Introduction
The Vitamin K Dependent [VKD] clotting factors involved in coagulation can be divided into:
1. Procoagulant - they are involved in the generation of a Fibrin clot
Factor II – Prothrombin
Factor VII
Factor IX
Factor X
2. Antithrombotic - they regulate coagulation
Protein C
Protein S
3. Others
Protein Z
There are a number of other Vitamin K [VK] dependent proteins e.g. Osteocalcin - that are not involved in coagulation – see references for further information.
| VK-dependent Proteins | Function | Gene Location |
|---|---|---|
| Procoagulant | ||
| Factor II [Prothrombin] |
Generation of Thrombin [IIa] and which has multiple functions. Prothrombin is the most abundant vitamin K dependent protein in plasma. |
F2 11p11.2 |
| Factor VII | Initiates coagulation through its interaction with Tissue Factor [TF] and the subsequent cleavage/generation of FVIIa by different proteases including FIIa [Thrombin], FXa, IXa, XIIa and the FVIIa-TF complex. | F7 13q34 |
| Factor IX | Activated to FIXa by FXa or the TF-VIIa complex in the presence of Phospholipid [PL], Ca2+ and FVIII. FIXa activates Factor X to Xa. |
F9 Xq27.1 |
| Factor X | Activated by: FIXa and FVIIIa TF-VIIa complex to generate FXa and which forms part of the Prothrombinase complex that activates Prothrombin [FII] to Thrombin [IIa]. |
F10 13q34 |
| Anticoagulant | ||
| Protein C | Protein C is activated by Thrombin to Activated Protein C [APC] in a reaction that is accelerated by the endothelial receptor, Thrombomodulin. Activated Protein C [APC] is involved in the inactivation of Factors Va and VIIIa but has additional roles in the regulation of inflammation and apoptosis. | PROC 2q14.3 |
| Protein S | Protein S acts as a cofactor for APC in the inactivation of Factors Va and VIIIa. Protein S is also involved in cell apoptosis and in the regulation of Complement activation. |
PROS 3q11.1 |
| Protein Z | Protein Z serves as a cofactor for the inhibition of FXa by the Protein Z-dependent Inhibitor [PZI]. Protein Z-dependent Protease Inhibitor (ZPI) is a 72-kDa protein and a member of the SERPIN superfamily of proteinase inhibitors that produces rapid inhibition of FXa in the presence of Protein Z [PZ], Phospholipid and Ca2+. The rate of FXa inhibition by ZPI is reduced >1000-fold in the absence of PZ. |
PROZ 13q34 |
Studies have shown that mutations in the genes encoding the proteins involved in Haemostasis may lead to the development of a bleeding disorder but in rare cases they may also lead to the generation of a pro-thrombotic state and thrombosis. For example, a spectrum of mutations within the F9 gene encoding the Factor IX protein, have been reported and are associated with low Factor IX levels and an increased risk of bleeding - Haemophilia B. In contrast, Factor IX Padua [Arg338Leu] a missense mutation in the F9 gene, is a gain of function mutation that leads to an 8-fold increased specific activity of the Factor IX protein and is associated with an increased risk of venous thrombosis. Similarly mutations in the F2 gene encoding Factor II [Prothrombin] may lead to a bleeding disorder but a small number of mutations primarily located within Exon 12 of the F2 gene lead to a thrombotic phenotype. These mutations appear to interfere with the inactivation of Thrombin [IIa] by Antithrombin leading to the persistence of Thrombin in the circulation and an increased risk of thrombosis.
1. Factors II [Prothrombin], VII, IX, Protein S and Protein Z circulate as single-chain molecules. Factor X and Protein C circulate as two-chain molecules in which the two chains are linked by a disulphide bond.
2a. Factors VII, IX, X and Protein C have a similar domain structure comprising:
- an N-terminal Gla domain followed by
-
two Epidermal Growth Factor [EGF]-like domains [EGF-1 and EGF-2]
- a C-terminal serine protease domain
b. Protein Z has a similar domain structure but it contains a C-terminal pseudoprotease domain that has no catalytic activity.
3. Protein S consists of :
- an N-terminal Gla domain
-
a Thrombin-Sensitive Region [TSR]
-
four EGF-like domains [EGF1 - EGF-4]
-
a sex hormone globulin binding domain.
4. Prothrombin [Factor II] has a distinct domain structure, in which the N-terminal Gla domain and the C-terminal protease domain are linked by two kringle domains. The Kringle domains replace the EGF-like domains. Kringle domains [named after their resemblance to a Scandinavian pastry] are conserved sequences that fold into large loops and are stabilised by disulphide bonds.
SERINE PROTEASES: Serine proteases are enzymes that cleave specific peptide bonds in a protein. This activity is dependent upon a series of amino acids located in the active or catalytic site of the molecule, one of which is always a serine residue and hence the name.
SERPINS: SERine Protease INhibitorS [SERPINS] such as Antithrombin act as a pseudo-substrate for the serine protease. The serine protease recognises and then cleaves a peptide bond in the inhibitory protein [the SERPIN] that leads to a conformational change in the structure of the SERPIN and which then locks the serine protease into an inactive complex that is then removed from the circulation.
| VK-dependent Protein | Domains | Comments |
|---|---|---|
| Procoagulant | ||
| Factor II [FII - Prothrombin] | Gla Domain Kringle Domain 1 Kringle Domain 2 Serine Protease Domain |
The activation of Prothrombin [II] to Thrombin [IIa] is summarised at the end of this page. |
| Factor VII [FVII] | Gla Domain EGF- like Domain 1 [EGF-1] EGF- like Domain 2 [EGF-2] Serine Protease Domain |
Factor VII is activated to VIIa by cleavage of the peptide bond between residues Arg152-Ile153. This cleavage by FXa, FIXa and the TF-VIIa complex, leads to the generation of a two chain molecule comprising a Light chain [152 residues] and a Heavy chain [254 residues] linked by a single disulphide bond between residues Cys135 located in the EGF2 domain and Cys262 located in the serine protease domain. |
| Factor IX [FIX] | Gla Domain EGF-like Domain 1 [EGF-1] EGF-like Domain 2 [EGF-2] Activation Peptide Serine Protease Domain |
Activation of FIX requires cleavage at residues Arg145-Ala146 and Arg180-Val-181 generating FIXa and the release of a 35-residue activation peptide. Factor IXa has two chains - a Light chain [144 residues] consisting of the Gla, EGF1, and EGF2 domains and a Heavy chain [267 residues] comprising the active site serine protease domain. The Light chain and the Heavy chain are linked by a single disulphide bond between Cys137 located in the EGF2 domain and Cys279 located in the serine protease domain. |
| Factor X [FX] | Gla Domain EGF-like Domain 1 [EGF-1] EGF-like Domain 2 [EGF-2] Activation Peptide Serine Protease Domain |
Factor X is activated to Xa by cleavage of the peptide bond between residues Arg194-Ile195. This cleavage by FIXa-FVIIIa, or the TF-VIIa complex, leads to the generation of a two chain molecule comprising a Light chain [138 residues] and a Heavy chain [253 residues] linked by a single disulphide bond between residues Cys132 located in the EGF2 domain and Cys302 located in the serine protease domain. |
| Anticoagulant | ||
| Protein C [PC] | Gla Domain EGF-like Domain 1 [EGF-1] EGF-like Domain 2 [EGF-2] Serine Protease Domain |
Protein C circulates as a two-chain molecule comprising: Light Chain: 155 residues Heavy Chain: 254 residues The two chains are linked by a disulphide bond between Cys141-Cys277. Protein C is activated to Activated Protein C [APC] by cleavage at Arg169 by Thrombin. |
| Protein S [PS] | Gla Domain Thrombin-sensitive region [TSR] EGF-like Domain 1 [EGF-1] EGF-like Domain 2 [EGF-2] EGF-like Domain 3 [EGF-3] EGF-like Domain 4 [EGF-4] Sex Hormone Globulin Binding Domain |
PS circulates as a single chain molecule and functions as a cofactor for APC in the activation of Factors Va and VIIIa. PS also has APC-independent activity, acting as a cofactor for Tissue Factor Pathway Inhibitor [TFPI] and accelerating the inhibition of Factor Xa. |
| Protein Z [PZ] | Gla Domain EGF-like Domain 1 [EGF-1] EGF-like Domain 2 [EGF-2] Pseudoprotease Domain |
PZ circulates as a single chain molecule and is a cofactor for the serpin ZPI [Protein Z-dependent Protease Inhibitor]. PZ complexed to ZPI is involved in the regulation of both free FXa generated in the initiation phase of coagulation and Prothrombinase bound FXa. In this way PZ complements the regulation of the Prothrombinase complex by APC so reducing Thrombin generation A role for the PZ–ZPI complex in the regulation of pregnancy has been demonstrated. There is a progressive elevation in PZ levels in the first trimester of gestation, which then steadily declines toward delivery. An association between altered plasma PZ concentrations and adverse pregnancy outcomes (recurrent miscarriage, stillbirth, pre-eclampsia, intrauterine growth restriction and placental abruption) has been reported. |
The Vitamin K dependent factors require Vitamin K to generate a functionally active protein. Vitamin K originates from the German term - Koagulationsvitamin.
Vitamin K is fundamental to a post-translational modification [a Vitamin K-dependent carboxylation reaction] that converts specific glutamate residues [Glu] in the Vitamin K dependent protein, to gamma-carboxyglutamate residues [Gla] and the generation of a functionally active protein.
The Gla domain is located at the N-terminus of the Vitamin K dependent Protein – see the image below for Factor VII:
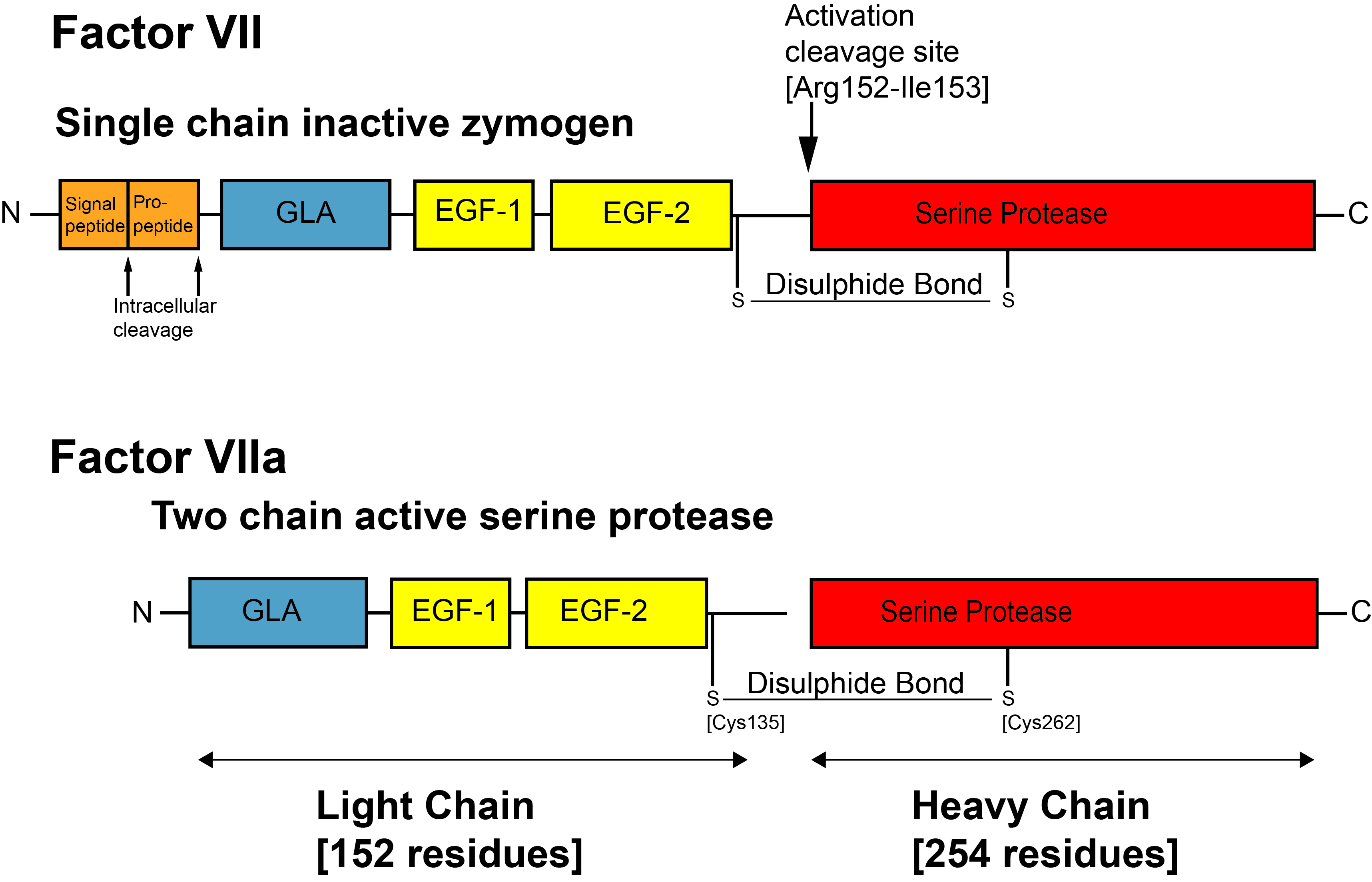
The N-terminal Gla domain is common to all of the Vitamin K dependent proteins and binding of Ca2+ and Mg2+ ions to the Gla domain induces a structural change in the molecule converting it from an unfolded to a folded protein. In this functional form, the Gla domain can bind to exposed Phospholipid [PL] membranes.
In addition to membrane binding, the Gla domains also function as cofactor and/or substrate binding recognition sites.
Vitamin K exists in two main forms: K1 and K2. Vitamin K3 is a synthetic form of Vitamin K.
K1: Phylloquinone
Found in green leafy plants and vegetables
K2: Menaquinone
Generated from bacterial synthesis e.g. in the intestinal flora and fermented foodstuffs
K3: Menadione
A synthetic, water soluble form. In many cases Vitamin K3 has been replaced by a synthetic form of Vitamin K1. Vitamin K3 is no longer used as a dietary/food supplement due to concerns about its safety in
humans.
Vitamin K is fundamental to a post-translational modification [a Vitamin K-dependent carboxylation reaction] that converts specific glutamate residues [Glu] in the Vitamin K dependent protein, to gamma-carboxyglutamate residues [Gla] and the generation of a functionally active protein.
The Gla domain is located at the N-terminus of the Vitamin K dependent Protein – see the image below for Factor VII:

The N-terminal Gla domain is common to all of the Vitamin K dependent proteins and binding of Ca2+ and Mg2+ ions to the Gla domain induces a structural change in the molecule converting it from an unfolded to a folded protein. In this functional form, the Gla domain can bind to exposed PL membranes.
In addition to membrane binding, Gla domains also function as cofactor and/or substrate binding recognition sites.
The carboxylation of the Vitamin K dependent proteins is fundamental to the generation of a functionally active protein. A deficiency of Vitamin K or drugs such as Warfarin which interrupt this pathway, leads to functionally inactive proteins.
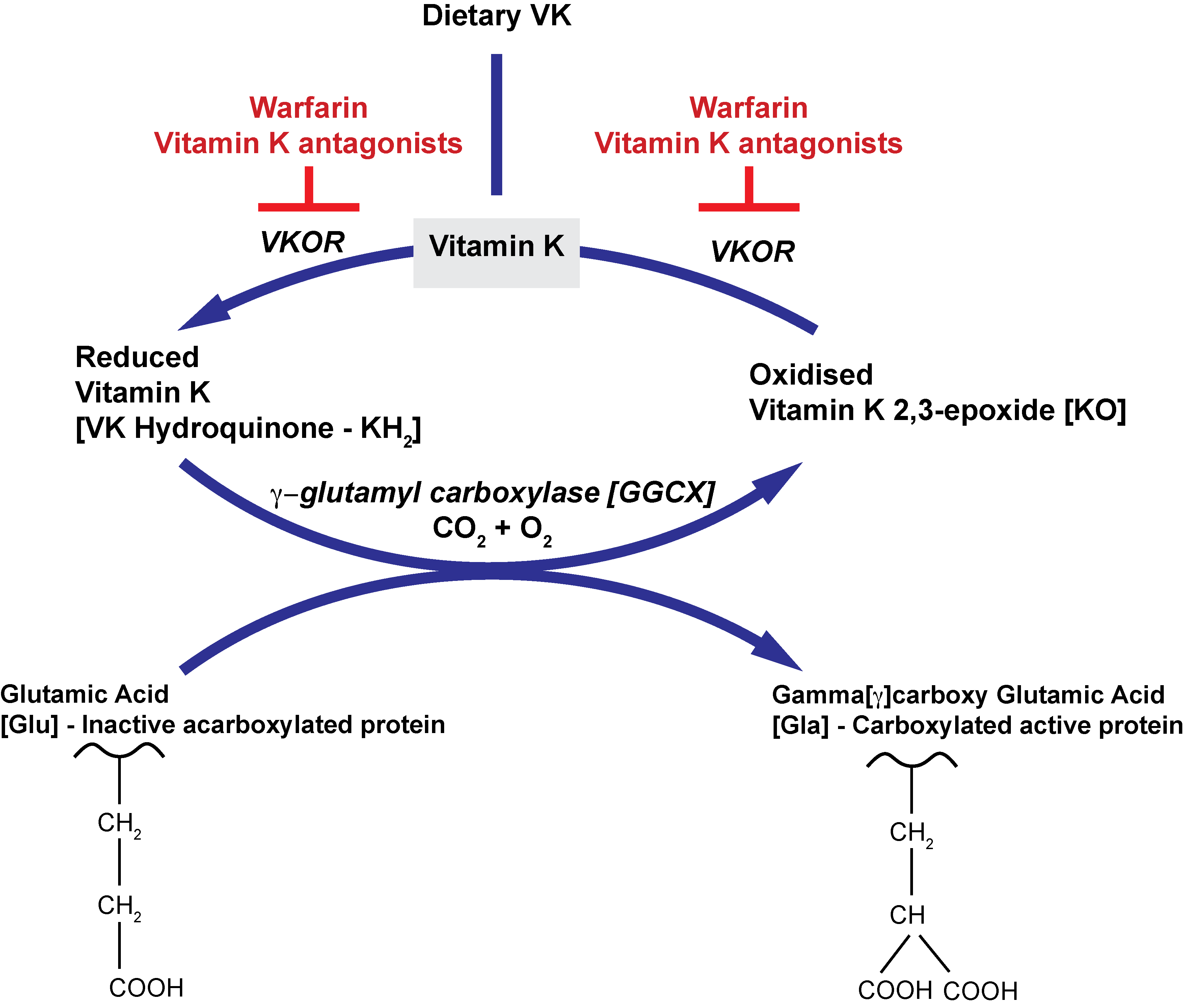
The biological functions of the Vitamin K-dependent clotting factors requires 9-13 Glutamic [Glu] residues located at the N-terminus of the mature protein (the Gla domain) to be modified by Vitamin K-dependent carboxylation. Carboxylation is catalysed by the membrane protein Gamma-glutamyl carboxylase [GGCX], which uses the reduced form of Vitamin K [KH2], CO2 and O2 as co-factors. The term ‘gamma’ arises because it is the gamma carbon atom in the Glutamic acid residue of the Vitamin K dependent protein, that undergoes the modification.
Simultaneously, the reduced Vitamin K [Vitamin K Hydroquinone or KH2] is oxidized to Vitamin K epoxide [KO] and it is this oxidation reaction that provides the energy required for the carboxylation reaction. The oxidised VK [KO] is converted back to KH2 by the enzyme Vitamin K epoxide reductase [VKOR]. It is this enzyme that is inhibited by Warfarin and other Vitamin K antagonists.
Gamma-glutamyl carboxylase recognizes its protein substrate by binding tightly to the propeptide of the substrate, which tethers the substrate to the enzyme. The propeptide of the VKD clotting factor appears to be essential for carboxylation and removal of the propeptide abolishes carboxylation. The propeptide of the precursor protein is removed after carboxylation to generate the mature, functionally active protein.
A rare bleeding disorder - Hereditary Deficiency of the Vitamin K-dependent Clotting Factors - results in under-carboxylation and decreased activity of the Vitamin K-dependent proteins. Two subtypes - Type 1 and Type 2 - have been reported and which arise from mutations in the genes encoding either:
Type 1: Gamma-glutamyl carboxylase [GGCX also known as VKCFD1] or
Type 2: VKOR [VKORC1].
The newborn infant has low Vitamin K levels due to:
i. Vitamin K is not transported efficiently across the placenta
ii. Human breast milk has low levels of Vitamin K although interestingly cows milk does not.
iii. The diet for the newborn infant does not contain green leafy vegetables or plants.
iv. The gut is sterile.
v. Poor intestinal absorption of vitamin K.
vi. Low activity level of vitamin K epoxide reductase in the newborn infant.
Vitamin K deficiency leading to bleeding in the newborn infant [termed Vitamin K Deficiency Bleeding [VKDB]] is classified by the time of presentation:
| Classification | Presentation | Aetiology |
| Early-onset VKDB |
0 - 24 hr following delivery | Rare Typically seen in infants whose mothers have been prescribed drugs that interfere with VK metabolism. These include: - Warfarin and other VK antagonists - Anticonvulsant drugs e.g. Phenytoin - Antituberculous drugs e.g. - Rifampicin, Isoniazid. - Some antibiotics e.g. cephalosporins It is also seen in women with malabsorption disorders or who are malnourished. In infants who do not receive VK prophylaxis at birth, the risk of VKDB is high [6-12%] and the prognosis is poor due to the high prevalence of intracranial haemorrhage. |
| Classic VKDB |
2 - 7 days | Arises due to: - A lack of of VK in breast milk - Maternal drug intake may also contribute - Inadequate VK prophylaxis at delivery - Idiopathic Classic VKDB has a good prognosis because bleeding is typically located in the gastrointestinal tract, nose, umbilical stump and skin. |
| Late-onset VKDB | 2 - 12 weeks |
This is seen in exclusively breast fed infants, infants with cholestasis and/or malabsorption leading to the poor absorption of vitamin K from the gut. Most reported cases of late-onset VKDB present with intracranial haemorrhage. |
The diagnosis of VKDB requires a Prothrombin Time [PT] ≥4 times the control value and at least one of the following:
1. A normal or increased Platelet count with a normal Fibrinogen and absent Fibrin Degradation Products [FDPs].
2. Normalisation of the prolonged PT after vitamin K administration.
3. Increased levels of PIVKAs [usually Factor II [PIVKA-II]].
PIVKAs are Proteins Induced by Vitamin K Absence and are the under or acarboxylated precursor proteins of the vitamin K-dependent clotting factors and are present in cases of Vitamin K deficiency or in individuals taking a vitamin K antagonist such as Warfarin. PIVKAs are synthesised in the liver and released into the blood but as carboxylation of the specific glutamate residues [Glu] in the GLA domain has not taken place, they are inactive. The levels of PIVKAs correlates with the severity of VK deficiency.
Vitamin K deficiency is rare in adults because the diet in most adults contains adequate amounts of Vitamin K1 and in part because the gastrointestinal flora synthesises Vitamin K2. However, certain disorders and some drugs can interfere with Vitamin K absorption, making it possible to become deficient. These include:
1. Ingestion of Vitamin K antagonists such as warfarin. Whilst not affecting the levels of VK they inhibit its action.
2.
Antibiotics. Some broad spectrum antibiotics may destroy the GI flora and lead to a reduction in the synthesis of Vitamin K2
3.
Malabsorption syndromes. Vitamin K is a fat soluble vitamin and disorders affecting fat absorption such as obstructive jaundice may lead to VK deficiency. Extensive resection of the small bowel may also affect VK absorption and this may include some forms of bariatric surgery although the data on this is still unclear.
4.
A diet that is deficient in vitamin K.
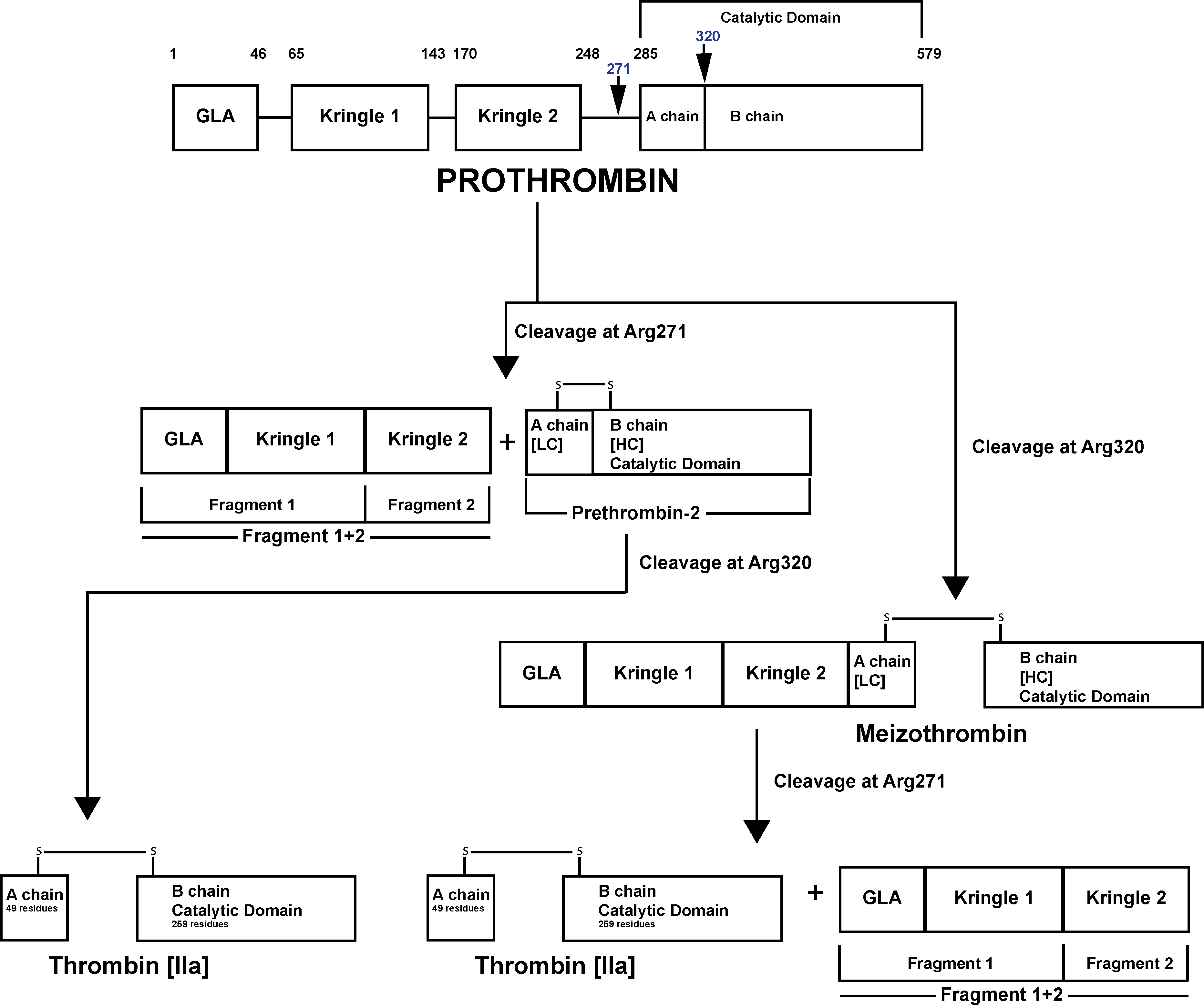
Prothrombin [Factor II] is activated to Thrombin [Factor IIa] by a membrane-bound enzyme complex - Prothrombinase and which is assembled through reversible interactions between Factor Xa and Factor Va on membranes containing Phosphatidylserine – a Phospholipid.
The Prothrombinase enzyme complex cleaves Prothrombin at two sites: Arg271-Thr272 and Arg320-Ile321 resulting in two pathways of Prothrombin activation:
1. Cleavage at Arg271-Thr272 generates Prothrombin Fragment 1+2 [F1+2] and the zymogen Prethrombin 2 [PT2]. Subsequent cleavage of PT2 by FXa at Arg320-Ile321 generates a two-chain active Thrombin molecule comprising a 49 residue A chain [the light chain] and a 259 residue B chain [the heavy chain] linked by a single disulphide bond.
2. Cleavage at Arg320-Ile321 generates Meizothrombin [mIIa]. Subsequent cleavage of Meizothrombin by FXa at Arg271-Thr272, generates Thrombin and F1+2.
Click HERE to return to the top of the page.
