Introduction
Factor VIII and Factor IX can be measured using a 1-stage assay but they can also be measured using other methods including the chromogenic assay. Chromogenic Factor VIII and Factor IX assays are becoming increasingly important as they address many of the problems associated with the monitoring of the extended half-life [EHL] clotting factor concentrates when measured using a 1-stage assay. In addition it is recognised that the 1-stage Factor VIII assay may over- or underestimate Factor VIII levels depending upon the underlying F8 gene mutation. The chromogenic Factor VIIII assay may therefore, correlate more accurately with the bleeding phenotype. It should be remembered, however, that many other factors affect the bleeding phenotype in an individual with Haemophilia A or B including the co-inheritance of other genetic mutations e.g. Factor V Leiden.
Principles & Methodology
Chromogenic assays involve the cleavage of a synthetic amidolytic substrate [or chromogen] which has been designed to contain a short amino acid sequence that is specific for the serine protease of interest. Activation of the inactive zymogen to a serine protease cleaves a chromophore [usually p-nitroaniline] from the C-terminus of chromogenic substrate generating a colour change which can be measured by its absorbance at 405nm. The change in optical density is proportional to the protease concentration although in some assays where an inhibitor is being measured, it is inversely proportional - see Antithrombin Assays.
Chromogenic substrate assays are relatively simple, inexpensive and readily automated. However, they may not be sensitive to all defects within a molecule e.g. in the GLA domain of the vitamin K dependent proteins - and so may give misleadingly normal results in some situations.
Chromogenic assays are also sensitive to a number of pre-analytical variables including elevated Bilirubin levels.
Chromogenic Factor VIII Assay
This is covered in elsewhere in the website. See 2-Stage Factor VIII Assays.
Chromogenic Factor IX Assay
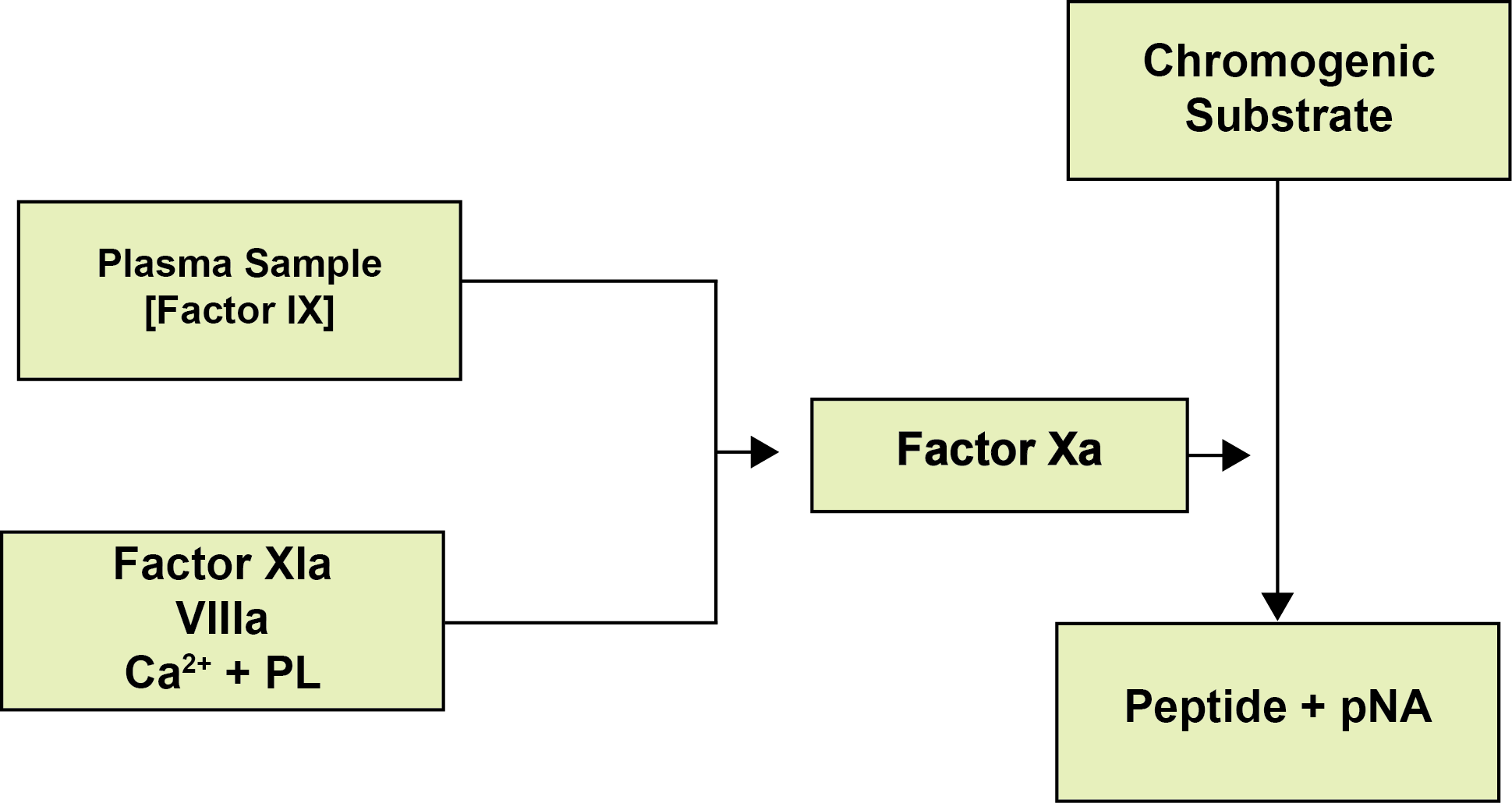
In the chromogenic Factor IX assay, Factor IX in the presence of Factor XIa is converted to the active serine protease Factor IXa. Factor IXa in the presence of Thrombin-activated FVIII [FVIIIa], Phospholipid and Calcium forms a complex that cleaves Factor X to Factor Xa. The amount of Factor Xa generated is measured using a specific chromogenic substrate the cleavage of which releases pNA leading to a colour change which is measured optically. The amount of pNA generated is directly proportional to the Factor IXa activity and as all the other reagents are in excess, the Factor IX concentration is the rate-limiting step and therefore the concentration of Factor Xa is a measure of Factor IX concentration.
Interpretation
See above regarding monitoring of EHL Factor IX concentrates and the chromogenic Factor IX assay.
In individuals with Haemophilia B when their Factor IX levels are measured using both a 1-stage Factor IX assay and a Factor IX Chromogenic assay no discrepancies are seen in individuals with severe Haemophilia B. However in individuals with a mild bleeding phenotype, higher Factor IX values using a chromogenic assays have been reported and many appear to be associated with specific mutations within the F9 gene [c.572G>A; p.Arg191His or c.571C>T; p.Arg191Cys] both of which are located at the N-terminal cleavage site of the activation peptide. The reported bleeding frequency for these individuals is low and indicative of a mild bleeding phenotype.
Reference Ranges
The Factor IX reference for adults is usually 0.50-1.50 IU/ml or 50-150 IU/dL.
Factor IX is a vitamin K dependent protein and the reference range, as for all the Vitamin K dependent clotting factors and natural anticoagulants, is different in the newborn infant. See Paediatric Reference Ranges for more information.
Click HERE to return to the top of the page.
