Introduction
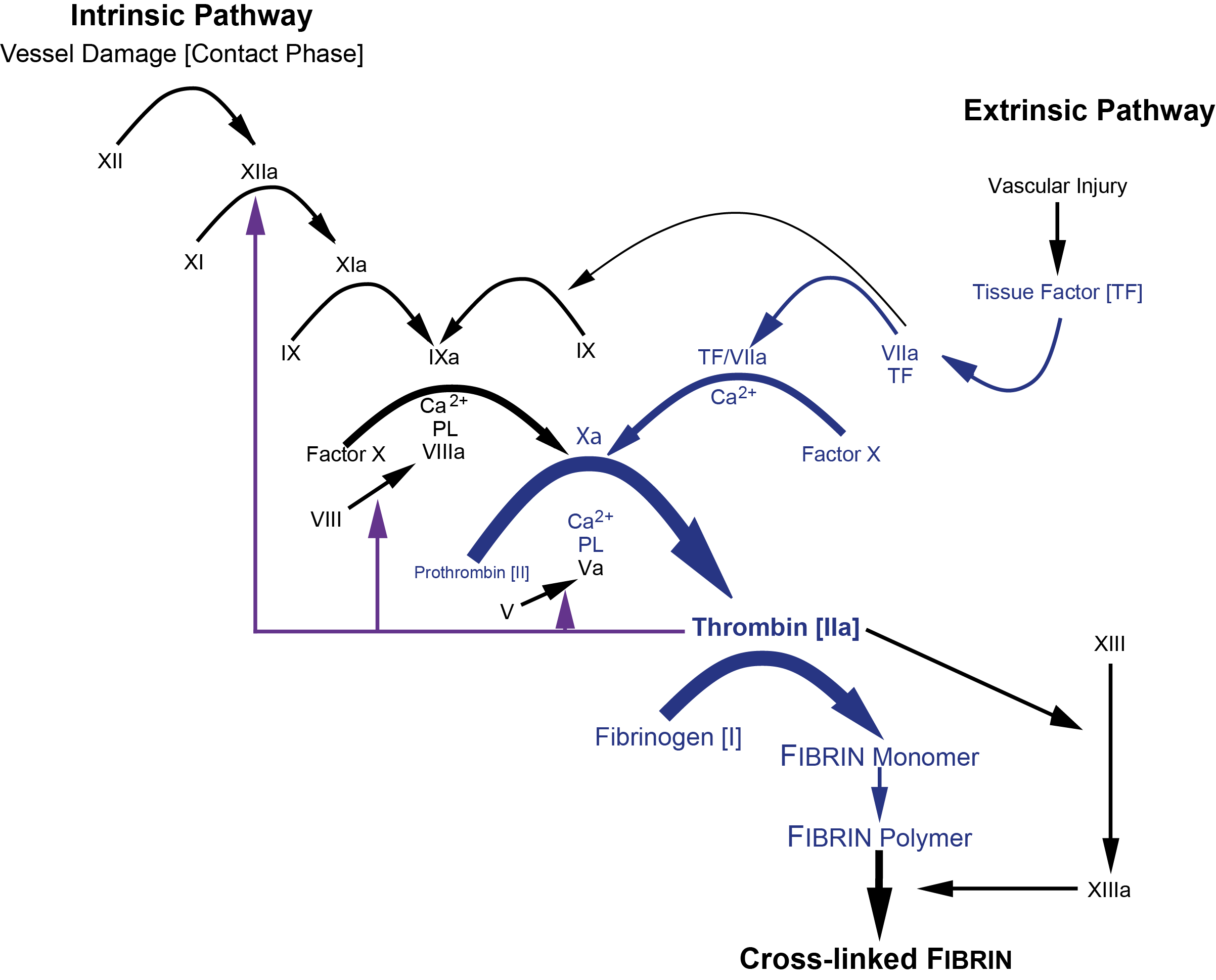
Active FXIII, [FXIIIa] functions as a transglutaminase and stabilises the fibrin blood clot by cross-linking the α- and γ-chains of Fibrin - see below. FXIIIa
also protects the newly formed Fibrin clot from plasmin-mediated fibrinolysis, primarily by cross-
linking α2-antiplasmin [α2AP] to Fibrin. Factor XIII has a number of other functions in addition to the stabilisation of the Fibrin clot and these include the maintenance of pregnancy, bone/cartilage growth, angiogenesis and wound healing.
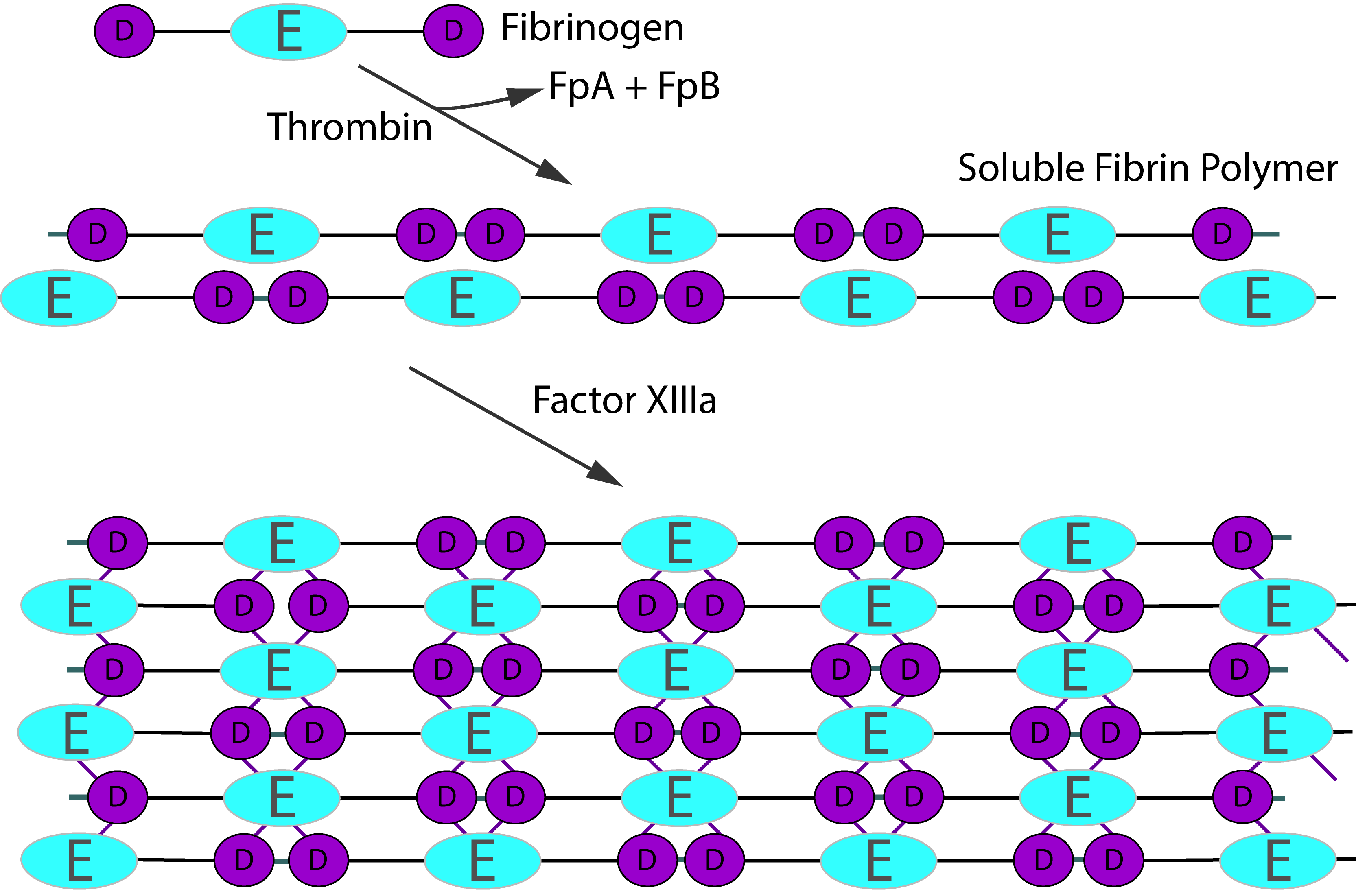
Plasma Factor XIII [pFXIII] is a tetramer composed of two A subunits [FXIII-A] and two B subunits [FXIII-B] held together by non-covalent bonds. In the circulation pFXIII is bound to the γ-chain of Fibrinogen. Intracellular Factor XIII, including platelet Factor XIII, comprises only the FXIII-A subunits and exists as a homodimer of the A subunit [FXIII-A2]. Factor XIII-A in platelets is present in concentrations approximately 150-fold greater than that in plasma. FXIII-A is produced in cells of bone marrow origin whereas FXIII-B is produced in the liver by the hepatocytes
pFXIII is activated by Thrombin [IIa] and Ca2+. Thrombin cleaves FXIII-A at Arg37-Gly38 releasing the 37-amino acid N-terminal activation peptide and this cleavage weakens the interaction between FXIII-A and FXIII-B, and in the presence of Ca2+ ions which bind to the Ca2+ binding site on the FXIII-A subunit, the inhibitory FXIII-B subunits dissociate. The activation of pFXIII is enhanced by polymerising Fibrin. The binding of pFXIII to newly polymerised Fibrin orientates pFXIII and Thrombin allowing Thrombin to cleave FXIII-A at Arg37-GLy38 and in the presence of Ca2+, the generation of a functionally active molecule [Factor XIIIa, FXIII-A2*]. FXIII-A2* remains bound to the newly formed Fibrin whereas the FXIII-B detaches from it. The conformationally active molecule has a number of active site cysteine residues which react with the Fibrin monomers catalysing their crosslinking.
At high Ca2+ concentrations [>100mmol/L] the A2B2 complex dissociates without prior proteolytic cleavage by Thrombin and the non-truncated FXIII-A2 molecule becomes an active molecule [FXIII-A2°]. This mechanism may be important in the activation of cFXIII in platelets and monocytes.
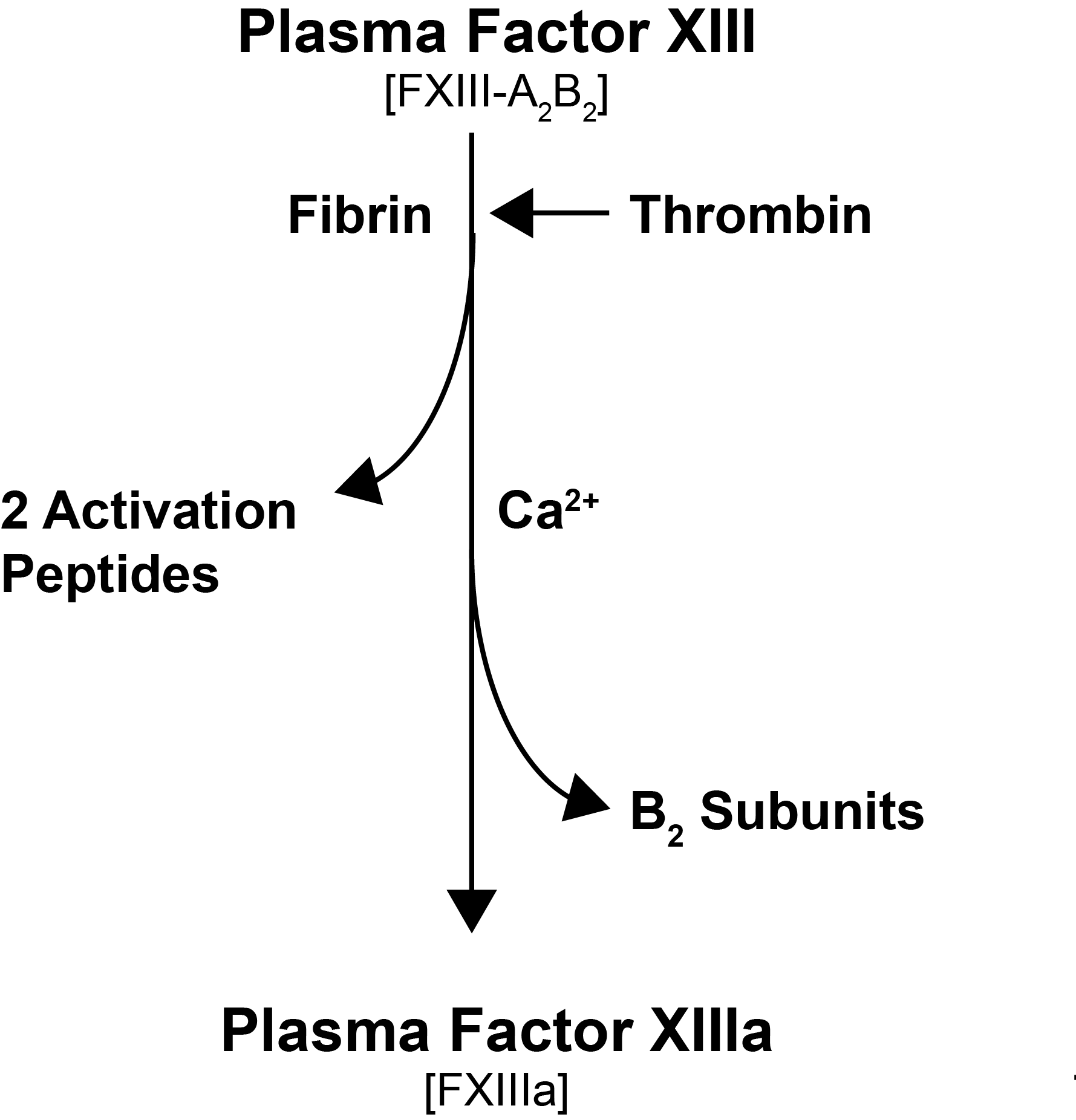
| Subunit | Structure/Gene |
|---|---|
| A subunit | The A subunit is the active part of the molecule and functions as a transglutaminase cross-linking the Lysine residue of one γ-chain to the Glutamine residue of another γ-chain to form cross-linked Fibrin – a transglutaminase reaction which releases ammonia. The gene for the FXIII-A subunit [F13A] maps to chromosome 6 [6p24-p25] and contains 15 exons encoding a 3.9kb mRNA. FXIII is activated by Thrombin in the presence of Calcium ions and Fibrin cleaving the activation peptide A. The B subunit then dissociates from the A subunit in the presence of Calcium and Thrombin and then the cleaved Factor XIIIa undergoes a conformational change exposing the active site which reacts with the fibrin monomers cross-linking the Lysine of one γ-chain to the Glutamine residue of another γ-chain to form cross-linked fibrin. Factor XIII-A contains the binding sites for Thrombin and Calcium that are required for catalytic activation. Factor XIIIa also forms covalent bonds with α2-Antiplasmin and TAFI [Thrombin Activatable Fibrinolysis Inhibitor] stabilising the fibrin clot and making it resistant to proteolytic degradation by the fibrinolytic pathway. Factor XIII is also found in platelets and monocytes/macrophages but as a dimer [FXIII-A2] and not complexed to FXIII-B2. |
| B subunit | The B subunit has no enzymatic activity and functions as: 1. A carrier for the A subunit preventing its proteolytic degradation within the plasma 2. In the binding of FXIII to the fibrin clot. The gene for the FXIII B subunit [F13B] maps to chromosome 1 [1p31-q32.1] and consists of 12 exons encoding a 2kb mRNA. |
Factor XIII Nomenclature
| Factor XIII | Nomenclature |
|---|---|
| Factor XIII in general without specifying its form | FXIII |
| Plasma FXIII | pFXIII and which circulates in plasma as a tetramer comprising FXIII-A2B2 |
| Cellular FXIII [Platelets, mekakaryocytes, monocytes, macrophages] | cFXIII |
| FXIII Subunit A monomer | FXIII-A |
| FXIII Subunit A dimer | FXIII-A2 |
| FXIII Subunit B monomer | FXIII-B |
| FXIII Subunit B dimer | FXIII-B2 |
| Factor XIII-A gene | F13A1 |
| Factor XIII-B gene | F13B |
| Activated FXIII | FXIIIa |
| Activation Peptide cleaved from FXIII by Thrombin | AP-FXIII |
| Inactive Intermediate FXIII formed from cleavage of FXIII by Thrombin in plasma or cellular | pFXIIIa' cFXIIIa' |
| Inactive intermediate FXIII A subunit from which the Activation Peptide has been cleaved by Thrombin | FXIII-A' - as a monomer FXIII-A2' - as a dimer |
| Thrombin and Ca2+ activated FXIII | FXIIIa* |
| Active A subunit in FXIII activated by Thrombin and Ca2+ | FXIII-A* - as a monomer FXIII-A2* - as a dimer |
| FXIII activated by Ca2+ but without proteolysis | FXIIIa° |
| Active A subunit in FXIII activated by Ca2+ but without proteolysis | FXIII-A° - as a monomer FXIII-A2° - as a dimer |
| Recombinant cellular FXIII | rcFXIII |
A. Clot Solubility Assays
Laboratories may use a clot solubility test as a screening test for Factor XIII deficiency and only if this is abnormal, is a formal FXIII assay performed. However, whilst the assay is easy to perform and relatively inexpensive, the results can be misleading and may fail to identify mild-moderate Factor XIII deficient patients [Factor XIII >2-3 IU/dL]. In addition, Hypofibrinogenaemia and Dysfibrinogenaemias can lead to a false positive test and these should be excluded before a clot-solubility test is undertaken. Whilst not a recommended test, the use of two different clot solubility tests run in parallel may be of value when other tests are not available.
Clot-solubility tests that use added Thrombin have been reported to have greater sensitivity than other solubility tests. The recommendations are not to use the Clot Solubility Assay for the diagnosis of Factor XIII Deficiency.
Quantitative FXIII activity assays are recommended as first-line screening tests whenever possible due to the insensitivity of the Clot Solubility Assay and the lack of standardisation. The Clot Solubility Assay cannot be used to monitor Factor XIII replacement therapy.
| Screening Tests | |
|---|---|
Urea Method |
The plasma samples [patient and control] are clotted by the addition of an excess of calcium and incubated at 37°C for 30 minutes. An alternative approach involves clotting the plasma with Thrombin and saline. The clot is removed and placed in 5M urea and incubated for 24 hours at either room temperature or 37°C. If the clot has dissolved this suggests FXIII deficiency and a formal FXIII assay should be undertaken. Hypofibrinogenaemia and Dysfibrinogenaemia can cause false-positive results and a functional Fibrinogen assay, Thrombin time/Reptilase time should be performed to exclude these disorders before the Urea screening test is performed. |
Acetic Acid |
The plasma samples [patient and control] are clotted by the addition of an excess of calcium and incubated at 37°C for 30 minutes. An alternative approach involves clotting the plasma with Thrombin and saline. The clot is removed and placed in either 2% Trichloroacetic acid or 1% Monochromatic acid and incubated for 24hours at either room temperature or 37°C . If the clot has dissolved this suggests FXIII deficiency. |
2. Immunological Assays
A number of assays exist for the measurement of the FXIII-A and FXIII-B subunits and the FXIIIA2B2 complex, by ELISA. In general the FXIII-A subunit is assayed first and if it is low then the B subunit is measured. These assays may fail to detect rare qualitative [Type II] variants in which the immunological Factor XIII levels are normal but the protein is functionally defective. In these rare cases a functional FXIII assay is needed and for these reasons, immunological FXIII assays cannot replace, functional FXIII assays. An immunological FXIII assay is required for the classification of FXIII deficiency.
a. ELISA Assays for FXIII
A number of assays exist for the measurement of both the FXIII-A and FXIII-B subunits and these include Electroimmunoassay [EIA], Latex immunoprecipitation assays and ELISA assays. In general the FXIII-A subunit is assayed first and if it is low the B subunit is measured as the B subunit will not be low with a normal A subunit but the converse is not true. ELISA assay are available to measure FXIII-A, FXIII-B and the FXIII-A2B2 complex and are more widely used than other immunological assays.
b. Laurell Electroimmunodiffusion
The Laurell Immunodiffusion technique can be used to measure FXIII although in practice it rarely is. The agar plate contains an antibody to FXIII and after electrophoresis a rocket shaped precipitin pattern forms along the axis of migration that can be visualised after staining with Coumassie Blue. The height of the peak (the ‘rocket’) is proportional to the concentration of FXIII.
3. Functional FXIII Assays
There are a number of methods for measuring Factor XIII levels and which rely upon the transglutaminase activity of FXIIIa. In general, these quantitative activity assays are based on two principles:
1. The measurement of Ammonia [NH4+] released during the transglutaminase reaction
2. The
measurement of a labelled amine incorporated into a protein substrate
a. Ammonia release assay:
Factor XIII is activated by Thrombin and Ca2+. Soluble fibrin generated by the Thrombin accelerates the reaction but an inhibitor of fibrin polymerisation is contained in the assay and which prevents the formation of a fibrin clot. The Factor XIIIa transglutaminase activity between a synthetic amine peptide substrate and a Glutamine [Gln] containing oligopeptide leads to the generation of Ammonia [NH4+]. The generation of ammonia is continuously monitored through the action of Glutamate Dehydrogenase [GLDH] that converts NADPH to NADP+. The conversion of NADPH to NADP+ can be monitored by its absorption at 340nm. Ammonia release assays are rapid, readily automated and easily standardised. However, a plasma blank is required in the assay to correct for FXIIIa-independent ammonia release and which is required to avoid incorrect results in the low activity range [<5-10%].
b. Amine incorporation assay:
In this assay a high dilution of the plasma sample prevents the formation of fibrin polymers and in addition the use of various peptides inhibits fibrin polymerisation. In these assays the binding of a labelled acyl acceptor substrate amine to an acyl donor glutamine residue of a protein by FXIIIa is measured.
in general the Amine incorporation assays are time consuming and difficult to standardise.
c. Isopeptidase assay:
In specific conditions FXIIIa can exert isopeptidase activity i.e. it can release amines bound to a Gln residue in an oligopeptide. This release can be measured by the use of a N-terminal coupled dye and the florescence is proportional to Factor XIII activity.
5. Chromogenic FXIII Assays
Chromogenic assays are available for measuring FXIII [actually FXIIIa] based upon its transglutaminase activity. The tests are based upon the incorporation of a biotinylated amine substrate [5-(biotinamido)pentylamine] into Fibrinogen [immobilised on a microtitre plate] by Thrombin activated FXIII[a]. The incorporated amine substrate is detected with a strepavidin-enzyme conjugate and chromogenic substrate. The colour change is proportional to the concentration of FXIIIa.
6. Thromboelastogram
The TEG can also detect significant FXIII deficiency – the TEG shows reduced clot formation and increased Fibrinolysis.
7. Factor XIII Inhibitor assays
Factor XIII inhibitors are rare but have been reported. They may arise for a variety of reasons - see below. Approximately 50% of cases are idiopathic. Autoimmune FXIII deficiency occurs mainly in older adults.
Acquired FXIII deficiency can be broadly divided into:
1. Immune: Immune-mediated FXIII deficiency due to the presence of an autoantibody targeting FXIII epitopes on the FXIII-A subunit and which may lead to a severe bleeding phenotype. The clinical presentation is variable from mucocutaneous and intramuscular bleeding to life-threatening haemorrhages including intracranial, intra-thoracic or intra-peritoneal bleeds.
The bleeding phenotype in acquired immune-mediated FXIII deficiency does not always correlate with FXIII level or the inhibitor titre.
Neutralising autoantibodies against the FXIII subunit A [FXIII-A2] are usually detected by mixing studies:
| Tube | Composition |
|---|---|
| Tube 1 Patient |
Patient: A 1:1 mixture of the patient’s plasma and buffered pooled normal plasma |
| Tube 2 Control |
Control: A 1:1 mixture of buffered pooled normal plasma and FXIII deficient plasma |
The samples are Incubated for 2 hours at 37°C and the residual FXIII activity in both tubes is then assayed. The percentage residual FXIII activity is obtained by:
i. Deriving the corrected FXIII activity of the patient sample. This is obtained by:
Control Plasma FXIII activity [Tube 2] minus Patient Plasma FXIII activity [Tube 1]
ii. Derive the residual percentage FXIII activity. This is obtained by:
Corrected FXIII activity of the patient sample/FXIII activity of the normal and FXIII deficient plasma mixture and the result is multiplied by 100 to obtain the percentage residual FXIII activity
If the residual percentage FXIII activity is <75%, this raises the possibility of the presence of a neutralising antibody. A formal Bethesda assay can then be performed in which a series of serial dilutions of patient’s plasma is made and the dilution required to obtain 50% inhibition of the FXIII activity in the normal plasma is determined be and the results are commonly expressed in Bethesda units [Bu].
2. Non-Immune: Non-immune causes of FXIII deficiency appear to be more common than immune-mediated causes, rarely cause bleeding and are related to excessive consumption or decreased production of FXIII - see below.
Binding assays can detect non-neutralising antibodies by measuring the binding of patients’ immunoglobulins to purified plasma FXIII and purified FXIII subunits in an ELISA assay.
8. Evaluation of Fibrin cross-linking by FXIIIa by SDS PAGE
The cross-linking of Fibrin by Factor XIIIa can be investigated by SDS PAGE [sodium dodecyl sulfate-polyacrylamide gel electrophoresis].
The patient’s plasma is clotted by the addition and CaCl2 and Thrombin. The reaction is incubated for 60 minutes at 37°C and the activity of FXIIIa is inhibited by the addition of Iodoacetamide. The clot is removed and washed in NaCl containing Iodoacetamide. The washed clot is then dissolved in SDS buffer and analysed by SDS-PAGE. In parallel studies the transglutaminase activity of FXIIIa is prevented by the addition of Iodoacetamide to the CaCl2. solution.
SDS PAGE can also be performed on platelet lysates to analyse the FXIII-A2 (dimer) in platelets.
See References for additional information.
Interpretation
Low Factor XIII Levels are seen in:
- Individuals with inherited FXIII deficiency
- Factor XIII levels fall in pregnancy. Severe inherited FXIII deficiency is associated with recurrent miscarriage
- Excessive activation, as seen in DIC, exposure to some snake venoms and caterpillar toxins [Lonomia Achelous caterpillar Venom]
- Henoch-Schoenlein purpura (HSP)
- In patients on and following cardiopulmonary bypass
- Chronic inflammatory bowel disease
- Severe GvHD of the gut
Acquired Factor XIII deficiency secondary to an auto-antibody is rare and approximately 50% of cases are idiopathic. Such cases may arise due to an an autoantibody targeting FXIII-A, leading to an inhibition of FXIII activation or FXIIIa activity. Antibodies to FXIII-B have also been reported and these accelerate the the elimination of FXIII leading to low levels.
Other causes include:
- Autoimmune disease
- Malignancy
- Myelo and Lympho-proliferative disorders
- Various drugs including Isoniazid, Penicillin and Procainamide. In these cases the FXIII levels often return to normal after the drug is withdrawn.
For more information on Acquired Factor XIII deficiency see References: Ichinose A et al 2016 and Yan et al 2018.
Classification of Factor XIII Deficiency
The Factor XIII and Fibrinogen SSC Subcommittee of the ISTH has proposed a Classification for Factor XIII deficiency - see References: Kohler et al 2011.
Type I deficiency is a quantitative defect resulting from decreased synthesis of FXIII.
Type II deficiency is characterized by a normal or near-normal concentration of a functionally defective FXIII.
Diagnosis of Factor XIII Deficiency
The ISTH guidelines - see References and figure below - recommend that patients with suspected Factor XIII deficiency [based upon their clinical and family history] should be screened with a quantitative [functional] FXIIIa activity assay that has been shown to be able to detect all forms of Factor XIII deficiency.
In cases in which a low Factor XIIIa is found then additional tests should be undertaken to establish if this is quantitative or qualitative disorder and immunological testing is required to measure the levels of FXIII-A2, FXIII-B2 and FXIII-A2B2.
If Factor XIII levels are low then a screen for neutralising and non-neutralising antibodies should be performed. Evaluation of FXIIIa dependent Fibrin cross linking studies and can also be undertaken.
Finally, if Factor XIII deficiency is diagnosed then sequence analysis of the F13A1 and F13B genes should be performed both to establish the underlying genetic mutation[s] and to allow family studies to be performed. Factor XIIII deficiency can result from mutations of the A or B subunit genes but disorders of the A subunit gene are more common.

Reference Ranges
The concentration of subunit A in plasma is 60-130 U/dL and that of subunit B is also 60-130 U/dL. Much of FXIII circulates in plasma in association with Fibrinogen.
What Test Next?
Factor XIII deficiency is rare and in individuals in whom a low level is found, investigations should be undertaken to establish whether this is an acquired disorder or inherited. If appropriate, mutational analysis of both the F13A and F13B genes should be undertaken. Screening of family members is essential so that appropriate counselling can be given.
Consideration should also be given to the possibility that the low Factor XIII levels are acquired and the appropriate tests based upon the causes outlined under 'Interpretation' above, performed.
Click HERE to return to the top of the page.
