What is Quality Assurance?
Quality Assurance in the laboratory ensures that the result a laboratory generates and reports is accurate, precise and specific. External quality assurance (EQA) is an important component of the total quality assurance program of a clinical haemostasis laboratory. The same test, performed on the same specimen, should give the same answer wherever and by whoever it is carried out. In practice, of course, coagulation tests are biological-tests performed on blood samples and there will always be a certain amount of variation, but any individual test result should be similar to everyone else's. Internal Quality Control [IQC] and EQA [when available] should be a fundamental part of any test that a laboratory offers.
Why do we need Quality Assurance Schemes?
There are numerous examples in the literature that highlight the value of internal and external quality assurance schemes. Look at the following [fabricated] case study involving the measurement of the Prothrombin Time [PT] and the subsequent derivation of the INR. Fundamental to this measurement is a knowledge of the ISI of the Thromboplastin used in the PT assay. Remember the INR is calculated from the [PT ratio]ISI.
| PT Ratio of Test Sample | ISI = 1 | ISI = 1.2 | ISI = 1.5 | ISI = 1.8 |
|---|---|---|---|---|
| 2 | INR = 2.0 | INR = 2.29 | INR = 2.8 | INR = 3.5 |
| 3 | = 3.0 | = 3.74 | = 5.2 | = 7.2 |
| 4 | = 4.0 | = 5.28 | = 8 | = 12.1 |
It is clear that if a laboratory fails to recognise that with a new batch of reagents the ISI has changed and they continue to use the previous ISI, then potentially they may subsequently underdose or overdose their patients with a vitamin K antagonist e.g. Warfarin.
What is Quality Assurance [QA]?
There are 2 parts to QA:
- Internal Quality Assurance [IQA] or Internal Quality Control [QC] - 'Are my results today the same as yesterday?'
- External Quality Assurance [EQA] - 'Are my results the same as other labs performing the same test?'
Internal QC is the monitoring of any haemostatic test performed in the laboratory to ensure that there is no day-to-day or within the day variation. Commonly this involves includes a statistical analysis of the tests that have been performed and the use of control materials with an assigned value e.g. a Factor VIII standard. It is designed to ensure that there is continual evaluation of the reliability of any test that the lab generates.
External QA involves the evaluation of the performance of the laboratory in a particular test or tests by an external agency e.g. UK NEQAS. Such schemes are usually organised on a national or international basis and the analysis is retrospective with a comparison of labs using similar methodology against each other.
EQA and IQA - Definitions
There are some key definitions that you need to be aware of in relation to quality assurance:
| Definition | |
|---|---|
| Specificity | Measures only the analyte of interest |
| Accuracy | Closeness of agreement between the true value and the observed value |
| Precision | The closeness of agreement amongst a series of measurement of a single sample |
| Linearity | Ability to obtain results that are directly proportional to the [analyte] |
| Limits | Upper and lower limits of detection |
| Range | Interval between the upper and lower limits of detection |
| Robustness | A measure of how much a test is affected by small variations in methodology |
| Standardisation | A material standard or reference preparation is used to calibrate analytic instruments and to assign a quantitative value to calibrators. In some cases this can also refer to reference method which is an defined technique which provides sufficiently accurate and precise data for it to be used to assess the validity of other methods. These are often referred to as the 'Gold Standard' method. International Reference preparations e.g. WHO Standards - are not intended for routine use but designed to act as standards for assigning values to usually commercial ‘secondary standard’ or calibrators. |
| Reference Material | A reference standard is an analyte that that has been assayed and assigned a defined value usually by a commercial manufacturer or organisation e.g. WHO. These reference materials are not intended for routine laboratory use - see above. |
| Laboratory Controls | These are commonly commercial rather than 'in house' pooled plasma samples and are used to check for accuracy. Any test/assay should include controls of normal, high and low values in addition to including a calibrator. |
| Statistics | The common statistical formulae used in the laboratory include: SD: The Standard Deviation measures the degree of spread around the mean. 68.27% of all measurements will be within 1 SD of the mean, 95.45% within 2SD and 99.73% within 3 SD [approximately 95%, 95% and 99%.]. CV: The Coefficient of Variation is derived from [SD/mean] x 100 and so is expressed as a percentage. The CV is a commonly used measure of precision and a high CV indicates poor reproducibility in a test. |
The following examples demonstrate some of these principles. The results are replicate samples assayed at different time points.
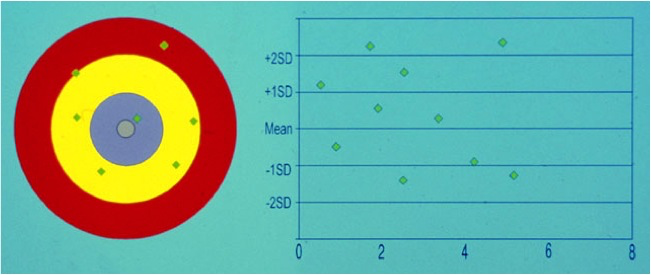 |
This test is both inaccurate and imprecise |
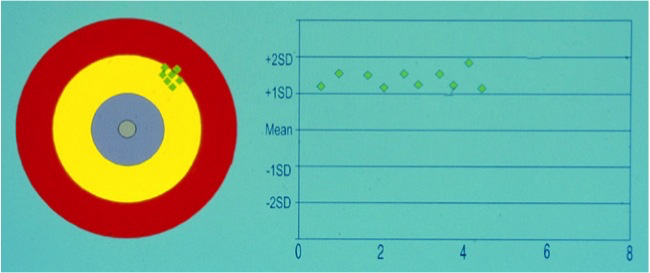 |
This assay is inaccurate but precise. The assay is said to have a positive ‘BIAS’ |
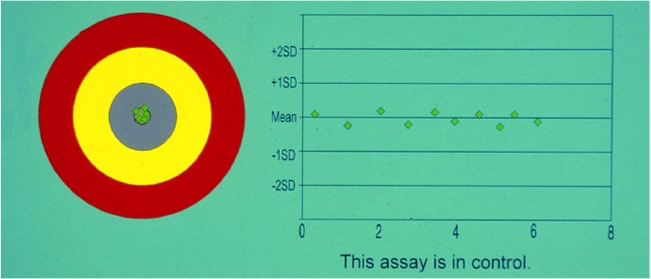 |
This assay is both accurate [i.e. no positive or negative bias] and precise [i.e. very little scatter of results about the mean value) |
Laboratory Standards & Controls
International Standards for use in the laboratory undergo calibration in extensive multicentre international collaborations and using multiple methodologies.
International Standards are usually limited in quantity and therefore used to calibrate National, Regional or Local Standards.
Remember the use of the following units have strict definitions and should not be used interchangeably:
'IU' = implies that an assay e.g. a FVIII assay - has involved the use of a reference plasma that has been calibrated against an International Standard with an assigned value in e.g. 100 International Units [IU] /dL or 1 IU/ml.
'U' = implies that an assay e.g. a FX assay - has involved the use of a reference plasma but this is not an international standard but it does have an assigned value e.g. Units [U] per ml - U/mL
'%' = implies that the assay has involved a reference plasma that has been derived from a normal plasma pool and which has been arbitrarily assigned a value of 100%.
Variables that can affect a Laboratory Test
Variables that affect a laboratory test can be summarised as:
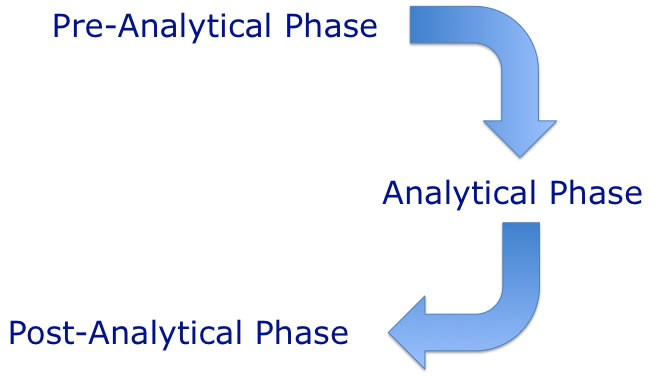
1. The Pre-analytical Phase - is discussed elsewhere on the website
2. The analytical variables include - processing of the sample in the lab, centrifugation speeds/times/temperatures, was the sample fresh or frozen, the assay methodology, the use of controls and standards and the equipment used to perform the assay.
3. The post-analytical variables includes - the preparation of the report, the interpretation of data and the transmission of results.
EQA Schemes
The following describes the UK NEQAS QA Scheme for Blood Coagulation. Similar schemes exist globally and the principles are very similar.
UK NEQAS Blood Coagulation [BC]
UK NEQAS is an NHS Not-for-profit organisation that is funded directly by participation fees. Currently NEQAS BC has some 800+participants in 30 different countries. It offers a number of schemes to participants. Samples are sent to laboratories, the individuals labs perform the relevant assays and return the results to NEQAS BC. The results are analysed and consensus results derived from all laboratories are generated to allow a comparison with both the overall median and the peer group median i.e. laboratories using similar assay methodology.
Assessing Performance
Performance is assessed depending upon the precise test:
1.
PT, INR and APTT: For these tests, a percentage deviation from the reagent median and overall medians is calculated and from this the following are derived:
| Within Consensus | Deviation <15% from reagent median [>10 users] or overall median [<10 users] |
| Outwith Consensus | Deviation >15% from the median |
| Persistently outwith consensus | Deviation >15% from reagent group or overall median on 3 consecutive occasions |
2. Factor assays: The percentage deviation is not used
because the spread of results is too large e.g. a factor VIII assay could generate results ranging from <1 IU/dL to >150 IU/dL. For these reasons an overall consensus median is determined as a central reference point and individual results are ranked into 5 unequal quantiles above or below the median. This is summarised in the illustration below:
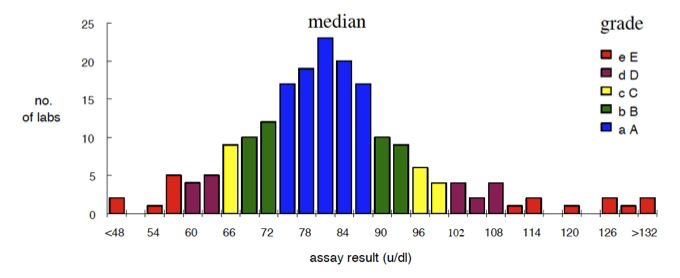
A/a: The nearest 25% above [A] or below [a] the median value [i.e. 50% of the results]
B/b: The next 10% above or below the median [i.e. 20% of the results]
C/c: The next 5% above or below the median [10% of results]
D/d: The next 5% above or below the median[10% of results]
E/e: The furthest 5% of results above or below the median [10% of results]
'A-E' refers to values above the median and 'a-e' to values below the median.
This approach identifies centres furthest from the median although there are some inherent problems with this methodology:
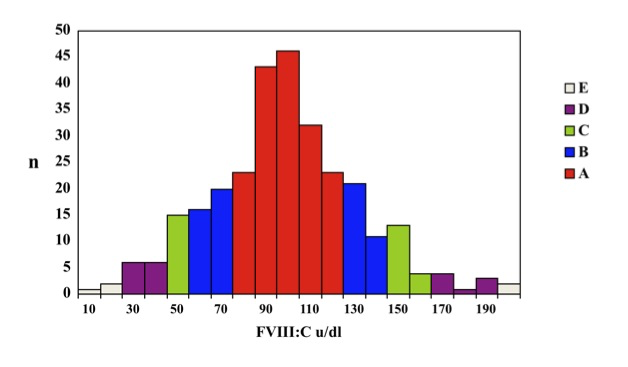
- For imprecise assays there will be a wide ‘acceptable’ range. For example, in an exercise involving measurement of VWF:RCo, an 'A' grade was awarded to any lab reporting a result between 63-96 U/dl.
- For more precise assays with a tighter range of results the acceptable range will be smaller. For example, in an exercise involving measurement of Factor X, an 'A' grade was awarded to any lab reporting a result between 46-52 U/dL.
- Finally because of this statistical approach 10% of labs in any test will get an 'E/e' grade.
The following example illustrates the results obtained for a FVIII assay and the distribution of grades:
Performance 'Outwith Consensus' and 'Persistently Outwith Consensus'
The terms 'outwith consensus' and 'persistently outwith consensus' refer to the grades and cumulative grades [in a number of exercises] that a lab receives for a specific test.
For
Screening Tests [PT/INR/APTT]:
Outwith consensus is defined as >15% deviation from the mean.
Persistently outwith consensus is defined as 3 consecutive surveys that are outwith consensus.
For Factor Assays:
Outwith consensus is defined as any combination of
C/E or D/D or D/E or E/E in TWO consecutive surveys.
Persistently outwith consensus is defined as TWO consecutive “outwith consensus” performances e.g. D/D/D, e/c/e, D/e/E - from three consecutive performances.
Non-participation in a particular exercise if a laboratory is registered for that exercise, is graded 'F' and is taken as the equivalent of an 'E' grade.
For Laboratories that are assigned 'persistently outwith consensus' the head of the relevant laboratory/department will receive a letter from the Director of UK NEQAS BC with the offer of advice or assistance. Laboratories that continue to be 'persistently outwith consensus' will be referred to the National Quality Assurance Panel [NQAAP].
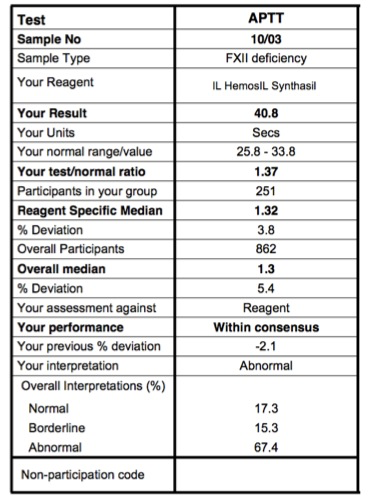
Case 1:
The following are the results from laboratory performing an APTT assay on a sample from a patient with Factor XII deficiency. Click HERE for an explanation of this report.
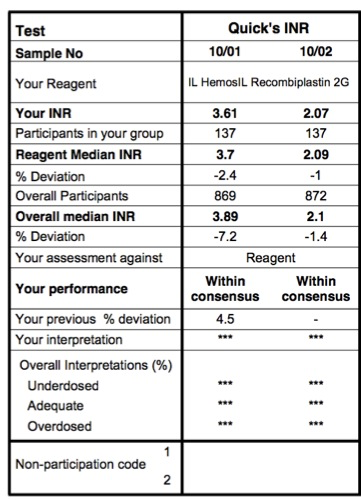
Case 2:
This is a NEQAS report of two INR results. The interpretation is very similar to that shown in Case 1.
Case 3:
This following shows a NEQAS return for a Factor VIII assay.
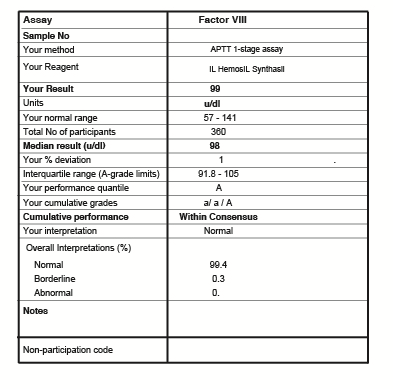
This report again is very similar to the previous 2 examples but the grading is given as a-e/A-E. The results for this lab for the FVIII assay was 99 U/dL with a median value of 98 U/dL.
50% of results in the exercise [25% below the median and 25% above the median] were within the range 91.8-105 U/dL and as this result for Lab participating in this exercise, was within this interquartile range at 98 U/dL, an 'A' grade was awarded.
In two previous exercises, this lab had been awarded 'a/ a' i.e. their results were within 25% of the median result for this assay but they were below rather than above and hence the lower case 'a.'
Remember, the grade 'A/a' relates to results that are 25% above [A] or 25% below [a] the median value [i.e. 50% of the results] and performance is based on two or more consecutive surveys.
Summary
In summary, IQA and EQA are fundamental parts of any laboratory test and this applies to Point of Care Testing [POCT] devices whether these are in a hospital setting or in the community and to molecular genetics. For more information - see References.
Acknowledgements
The support of UK NEQAS BC for permission to include their reports in this section is gratefully acknowledged.
