Introduction
Homocysteine [Hcy] is a sulphur containing amino acid that is derived from methionine, an essential amino acid found in abundance in protein of animal origin and the only source of Homocysteine in man. Homocysteine is metabolised through two separate pathways - the Remethylation pathway and the Transsulphuration pathway.
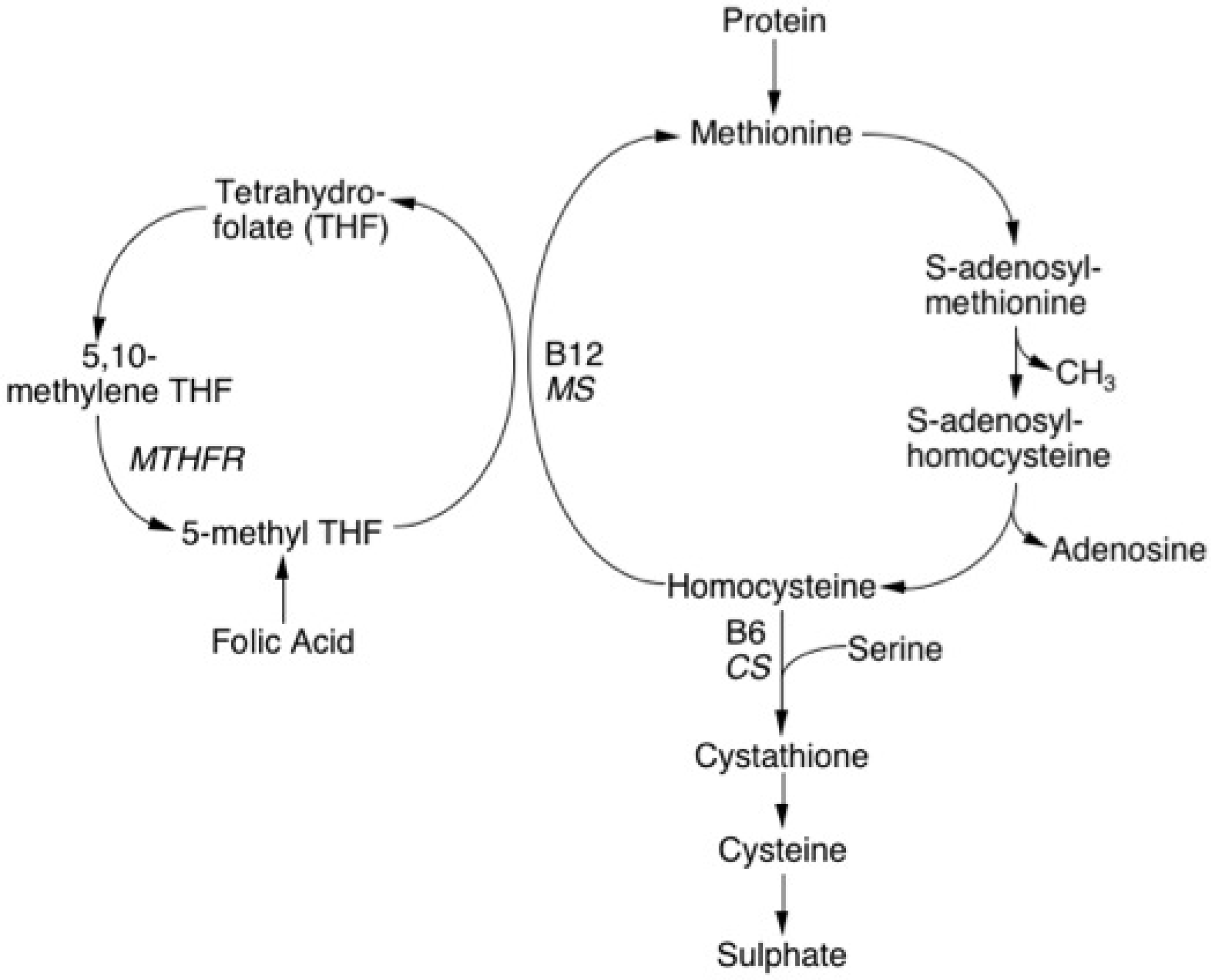
Remethylation Pathway: In the remethylation pathway, Homocysteine acquires a methyl group from either the conversion of 5-methyltetrahydrofolate to tetrahydrofolate or from the conversion of betaine to N,N-dimethylglycine. The former reaction is B12-dependent, occurs in all tissues and requires the enzyme methionine synthase (MS). The latter reaction, which in general is relatively minor, is B12-independent, occurs mainly in the liver and requires a different enzyme – betaine Homocysteine methyltransferase.
Homocysteine, which is not remethylated, is catabolised in a second pathway known as Transsulphuration.
Transsulphuration Pathway: In the Transsulphuration pathway, Homocysteine condenses with serine to form cystathionine, an irreversible reaction that is catalysed by the enzyme cystathionine-β-synthase (CβS) and which requires Pyridoxal-5'-phosphate (vitamin B6) as a co-factor. Cystathionine in turn is catabolised to cysteine and α-ketobutyrate by gamma-cystathionase. In the presence of excess methionine, transsulfuration is favoured with up-regulation of CβS and down-regulation of remethylation. Methionine is converted to Homocysteine via S-adenosylmethionine (SAM) which then serves as a methyl donor in a wide variety of biological reactions including purine and pyrimidine synthesis.
S-adenosylhomocysteine (SAH) the result of the demethylation of SAM is subsequently hydrolysed to Homocysteine and adenosine and re-starts the cycle of methyl group transfer.
Homocysteine exists in plasma in several forms:
| Form | |
|---|---|
| Free Homocysteine | The ‘free’ thiol (sulphydryl) molecule. Free unreduced Homocysteine is rapidly oxidised at physiological pH and is present in plasma in only trace amounts (<0.3nmol/L). |
| Homocystine | Homocysteine can be conjugated to another molecule of Homocysteine by disulphide bonding to form Homocystine. The oxidised fraction is largely composed of cysteine-homocystine which accounts for about 20-30% of total plasma Homocysteine. |
| Bound to other proteins | The majority of Homocysteine in plasma exists covalently linked via disulphide bonds to sterically unhindered peptide cysteine residues in various circulating proteins e.g. albumin and accounts for approximately 70-80% of total plasma Homocysteine. The sum of free and bound Homocysteine in plasma is denoted total Hcy and abbreviated ‘tHcy’. |
Principles & Methods
1. Cyanide-Nitroprussic acid Test: This is rarely if ever performed today. In classical Homocystinuria [HCU], excess Homocysteine disulphide present in the urine will react with a cyanide-nitroprussic acid solution to form Ferric Thiocyanate - an organometallic complex with a pink-purple colour. However, Homocysteine is extensively catabolised within the kidney and significant elevations in plasma Hcy may occur (>50 µmol/L) before Homocysteine is detectable in the urine.
2.
Assays for Homocysteine should measure plasma Homocysteine (i.e. the pure form), the mixed disulphide forms and the protein-bound forms. Plasma is, therefore, treated with a reducing agent e.g. Dithiothreitol [DTT] to cleave disulphide bonds prior to the measurement of Homocysteine. Methods to measure plasma Hcy include gas chromatography-mass spectroscopy, HPLC with or without fluorescence detection, HPLC and electrochemical detection, amino acid analyser detection and antibody fluorescence polarisation immunoassay.
3.
A recent development has been the development of a highly sensitive ELISA assay, which has considerably simplified the measurement of plasma Hcy.
4.
The Methionine Loading Test: The Methionine Loading Test (MLT) was introduced to detect heterozygosity for CβS deficiency – the key enzyme of the transsulphuration pathway. The test involves taking a standard oral dose of methionine (usually 0.1g/kg or 3.8g/m2) and then measuring plasma tHcy levels at a fixed time interval usually 4hr or 6h.r A 2hr post-MLT tHcy correlates well with the 4hr value and may be more practical in the clinical setting. An abnormal post-MLT tHcy is variously defined as any value above the upper value of the control group; any value greater than 2 standard deviations above the mean for the control group; or any value greater than the 90th percentile for the control group.
Several studies have shown that measurement of fasting tHcy alone, fails to identify some patients who have an abnormal response to an oral dose of methionine and who are at increased risk of vascular disease.
Historically the MLT has been thought to reflect the activity of the transsulphuration pathway, more recent data suggests that this is an over-simplification and that it may also be abnormal in individuals who have defects affecting the remethylation pathway.
5.
It is also possible to perform oral Homocysteine loading tests. L-Homocysteine thiolactone is used to prepare L-Homocysteine, which is then administered to the subject (65µmol/kg). The elimination of Hcy from the plasma is the followed for the next 24-72hr. The plasma clearance of Hcy is approximately 100ml/min, corresponding to a plasma half-life of approximately 3-4hr. Subjects with severe B12 or Folate deficiency appear to have normal tHcy clearance rates suggesting that their hyperhomocysteineaemia is due to an increased release of Hcy from the tissues rather to a reduction in its clearance. In contrast, individuals with renal failure have a markedly reduced clearance rate. The Homocysteine loading test appears to measure the rate of elimination of Hcy from the plasma whereas the MLT reflects intracellular Hcy formation, metabolism and its secretion into plasma.
Interpretation
Classical homocystinuria usually results from recessively inherited homozygous defects of either the CβS or MS enzymes involved in the Transsulphuration and Remethylation pathways respectively. Plasma tHcy levels in affected individuals are usually greater than 50µmol/L, often 200-400µmol/L and are associated with an elevated plasma methionine. Affected individuals display a variety of abnormalities including mental retardation, skeletal abnormalities and are at high risk of vascular disease.
A proportion of patients with classical homocystinuria due to deficiencies of CβS will respond to treatment with pyridoxine supplementation, with a reduction in plasma Homocysteine levels and a reduction in their subsequent risk of vascular disease. Heterozygous CβS mutations occur in approximately 0.5-1.5% of the general population and may be associated with mild elevations in plasma Homocysteine.
There are a number of factors that can influence plasma Hcy levels and these are summarised below.
Determinants of Plasma Homocysteine Levels
| Genetic | CβS deficiency MS deficiency MTHFR deficiency TL-MTHFR (C677T) |
| Physiological | Age: Homocysteine levels increase with age Sex: Pre- and postmenopausal women have lower tHcy levels than men Pregnancy: Levels fall Diet: tHcy levels directly related to methionine intake and inversely related to folate, B12, B6 and rarely choline, intake. Alcohol |
| Pathological | Vitamin deficiencies Renal disease: tHcy correlates with increasing creatinine and decreasing GFR Renal/cardiac transplantation: tHcy levels increased Severe psoriasis: Levels increased Leukaemia: Levels increased |
| Medications | Oral contraceptives/Hormone Replacement Therapy: levels reduced Steroids: Levels increased Ciclosporin: Levels increased Anti-folate drugs: Levels increased |
| Other | Smoking: Levels increased Nitrous oxide use as a 'recreational' drug can affect vitamin B12 activity leading to raised tHcy levels. |
1. Age, Sex, oestrogens and pregnancy: Plasma Homocysteine levels increase with age possibly because of an age-related decrease in the efficiency of the enzyme CβS. Dietary changes with increasing age may also be important.
Plasma Homocysteine levels are 25% higher in men than in pre-menopausal women – a difference that decreases but which does not completely disappear after the menopause. This may be due to differences in vitamin status between sexes, between different age groups and to the higher muscle mass in men.
Homocysteine levels are lower in women who take the oral contraceptive pill or hormone replacement therapy and there is a negative correlation between oestradiol levels and Homocysteine.
Homocysteine levels fall in pregnancy to approximately 60%of normal, probably because of a reduction in albumin, to which significant amounts of Homocysteine are bound. The development of hyperhomocysteineaemia in pregnancy appears to be prevented by an increase in the activity of the enzyme betaine Homocysteine methyltransferase.
Ethnic Variation: There appear to be some racial variations in the Homocysteine levels. Levels are lower in black South Africans than in White South Africans. This may in part, be explained by dietary differences and also in the prevalence of the thermolabile variant of the enzyme Methylenetetrahydrofolate reductase (MTHFR).
Diet: Plasma Homocysteine is directly related to methionine intake and levels are therefore lower in individuals who consume a diet low in protein of animal origin. Homocysteine levels are inversely correlated with vitamin intake and tend to be lower in diets rich in fresh fruit and vegetables. Alcohol tends to increase Homocysteine levels possibly through a direct action on folate metabolism or possibly because of an associated poor diet.
Pathological Vitamin Deficiencies: There is an inverse relationship between dietary Folate and vitamin B12 intake and Homocysteine levels. Homocysteine levels are lower in individuals whose diet is supplemented with vitamins. Elevated Homocysteine levels may occur in individuals with vitamin B12 or folate deficiency but no other evidence of an underlying megaloblastic anaemia.
Drugs: Homocysteine levels are increased in patients taking steroids, folate antagonists and ciclosporin.
Transplantation: In patients who undergo renal or cardiac transplantation, elevations in plasma Homocysteine may occur. In part this may be related to immunosuppressive agents or to a poor diet or a combination of both.
Renal Disease: Homocysteine levels are correlated with glomerular filtration rate (GFR) and serum creatinine through a mechanism that is not clear. Homocysteine levels are, therefore, increased in patients with renal failure. Folate supplementation of the diet in patients with renal disease, only partially corrects the elevation in Homocysteine even when large doses are administered. In patients who undergo peritoneal dialysis or haemodialysis, there is only a partial correction of the elevated Homocysteine. This may be due to the accumulation of inhibitors of folate metabolism which are only partially removed by dialysis. In individuals with normal renal function, virtually all the filtered Homocysteine is reabsorbed by the proximal tubule of the kidney and even in individuals with moderate elevations in plasma Homocysteine, there may very little if any in the urine.
Miscellaneous: Homocysteine levels are increased in patients with leukaemia and in patients with psoriasis. In both cases, treatment with the anti-folate drug Methotrexate, may be of importance. Homocysteine levels may also be increased in patients with sickle cell disease [SSD] but this may in part, be due to sub-normal vitamin B12 and Folate levels. Mild to moderate elevations in Homocysteine are also found in Polycythaemia vera and in Essential Thrombocythaemia but it does not appear to be an additional risk factor for thrombosis in these cases.
THERMOLABILE METHYLENETETRAHYDROFOLATE REDUCTASE (TL-MTHFR): Historically many cases of mild hyperhomocysteineaemia have been attributed to heterozygosity for mutations in CβS although this probably accounts for less than 1.5% of cases. In 1988, a thermolabile variant of the MTHFR (TL-MTHFR) enzyme was identified. The variant MTHFR exhibited in vitro thermolability at 46°C and a lower specific enzyme activity (50% of normal) in lymphocyte extracts and which allowed it be distinguished from the wild type enzyme. In contrast to the clinical and biochemical features seen in individuals with homozygous mutations affecting the MTHFR gene and resulting in classical homocystinuria, the TL-MTHFR variant produced no such abnormalities.
In 1994, the MTHFR cDNA was cloned and subsequent work revealed that the TL-MTHFR variant arises from a single nucleotide C→T substitution in the MTHFR gene at position 677 (C677T) resulting in the replacement of a highly conserved Alanine by a Valine. The mutation in the heterozygous or homozygous state correlates with reduced enzyme activity and increased thermolability in lymphocyte extracts and furthermore in vitro expression of a cDNA containing the mutation results in an enzyme that displays the variant phenotype
Reference Ranges
The normal reference range for Hcy is <16µmol/L. A level between 15 - 30 µmol/L is considered mildly elevated; 30 - 60 µmol/L moderately elevated and >60 µmol/L is considered severely elevated.
What Test Next
In individuals with an elevated Hcy, it is important to exclude deficiencies of folate or vitamin B12. Other causes should be considered by reference to the text and table above. Remember, the measurement of plasma Hcy is difficult and pre-analytical variables are very important. An abnormal result should be be confirmed on a repeat sample.
