Flow Cytometry
Introduction
Labelling platelets with antibodies directed against surface membrane glycoproteins and then analysing the binding by Flow Cytometry is a rapid and sensitive technique for the specialist haemostasis laboratory. Testing can be carried out in whole blood, PRP or washed platelets. Whole blood and PRP require small sample volumes and so is readily applicable to the neonate.
- - Flow cytometry can also be used in many settings including
- - Monitoring of GpIIb/IIIa antagonist therapy
- - Diagnosis of inherited deficiencies of platelet surface glycoproteins
- - Diagnosis of storage pool disease
- - Diagnosis of heparin-induced thrombocytopaenia
- - Diagnosis of Scott syndrome
.... but there are many more.
Whole blood flow cytometry overcomes many of the problems inherent in analysing PRP:
- - More physiological
- - Platelet activation is minimised as sample manipulation is minimal
- - Multiple platelet receptors can be analysed simultaneously
- - Novel antibodies can be readily incorporated into existing methodologies
- - Small volumes of blood required
- - Can be used in patients with significant thrombocytopaenia
Principles
This LINK takes you to a site that contains a tutorial on the principles Flow Cytometry.
Interpretation
The following images illustrate the role of flow cytometry in the analysis of Glanzmann's Thrombasthenia and Bernard Soulier Syndrome. In these two cases we have used three antibodies [CD41, CD61 and CD42b] directed against specific platelet membrane glycoproteins:
| Antibody | Specificity |
|---|---|
| CD41 | CD41 recognises the platelet membrane glycoprotein GPIIb (the integrin alpha IIb chain) which is non-covalently associated with GpIIIa (the integrin beta 3 chain) to form the GpIIb/IIIa complex. |
| CD61 | CD61 recognises the platelet membrane glycoprotein GpIIIa (the integrin beta 3 chain). |
| CD42b | CD42b reacts with GPIb on megakaryocytes and platelets. CD42b also inhibits ristocetin-dependent binding of Von Willebrand Factor to platelets and ristocetin-induced platelet agglutination. |
1. Normal Platelets: In Image 1, a normal blood sample – the platelets have been selected on the basis of forward and side light scatter and are circled in green. In image 2 the platelets have been stained with CD41 (GPIIb) and CD61 (GPIIIa) and express both these glycoproteins. Similarly in image 3, the platelets have been stained with CD42b (GPIb) and CD61 (GPIIIa) and express both these glycoproteins.
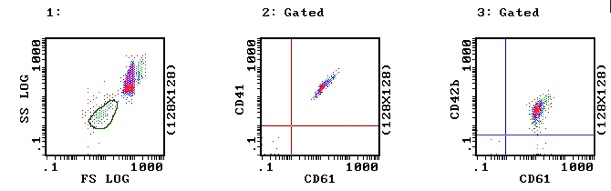
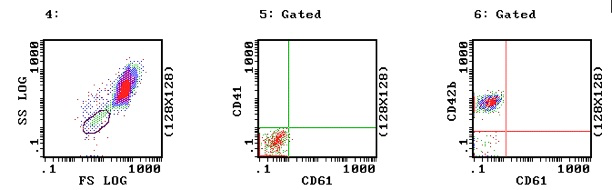
2. Glanzmann's Thrombasthenia: The following images are from a patient with Glanzmann's Thrombasthenia. In Images 5 and 6 the platelets show no activity with either CD41 or CD61 and so are missing the GPIIb/IIIa complex consistent with Glanzmann's Thrombasthenia. However, in image 6 they clearly show normal activity with CD42b i.e. normal GPIb.
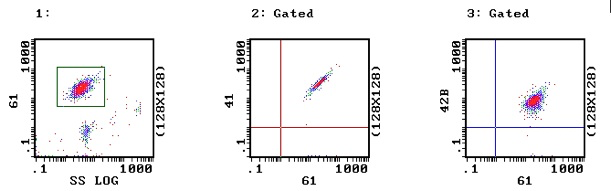
2. Bernard Soulier Syndrome
a. Normal Platelets: In Image 1 the platelets have been selected (gated) on the basis of side scatter and binding to CD61. In images 2 and 3 – the platelets (from a normal individual) show activity with both CD41 & CD61 (Image 2) and CD61 & CD42b (Image 3) indicating that the GPIIb-IIIa and GPIb receptors are present and that the antibodies are functioning.
b. The following images are from a patient with Bernard Soulier Syndrome. In Image 5 there is normal binding with CD41 & CD61 indicating a normal GPIIb-IIIa receptor complex but in Image 6 there are two pools of platelets – a pool with normal CD61 and CD42 binding (53% of all the platelets analysed) and a smaller pool of 47% which demonstrates normal CD61 binding but defective CD42 binding consistent with absence of the GPIb receptor. These platelets are from a patient with Bernard Soulier Syndrome following a platelet transfusion and the two pools represent both the patient’s platelets and the normal transfused platelets.
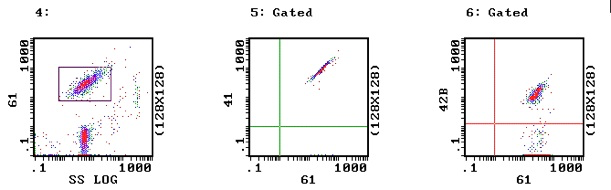
Interestingly (data not shown) the mean fluorescence for the BSS pool with CD61 is greater than the normal platelet pool and this reflects the elevated MPV and the presence of large platelets in BSS.
What Test Next
On the basis of these tests, one can make a diagnosis of either GTT or BSS. Additional tests would include gene sequencing to identify the causative mutation and family studies.
