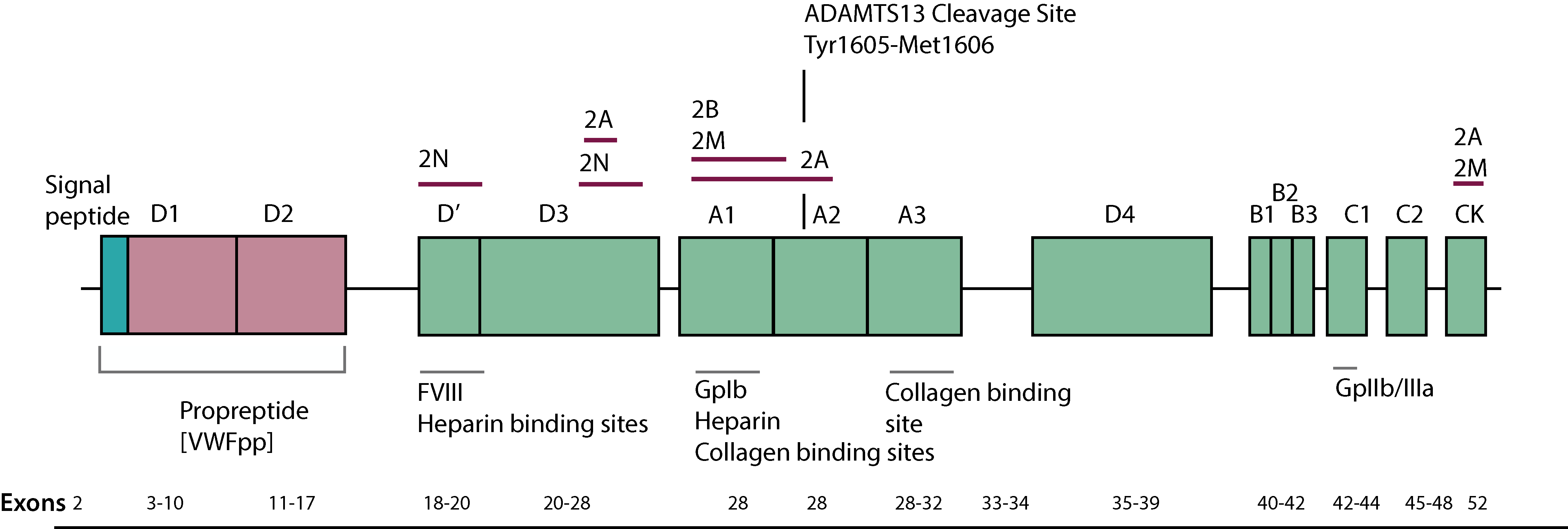
2. Von Willebrand Factor [VWF] Inhibitor Assays
1. Acquired dysfunction of von Willebrand Factor [VWF] - Acquired Von Willebrand Syndrome [AVWS]
An acquired dysfunction of von Willebrand Factor [VWF] arises in most cases from an increased clearance of VWF from the plasma and may occur for a number of reasons including:
i. The development of antibodies directed against functional or non-functional domains of the VWF protein.
ii. Non-specific antibodies which form complexes with VWF and which are then rapidly cleared from the circulation.
iii. Selective absorption of large and intermediate VWF multimers onto malignant cells or activated platelets.
iv. Proteolysis of VWF as it passes through a stenosed aortic valve or a ventricular septal defect [VSD]. High shear forces can induce structural changes in the shape of the VWF molecule, leading to exposure of the cleavage site for ADAMTS13. This results in cleavage of the highest molecular-weight multimers of von Willebrand factor, which are the most effective in platelet-mediated haemostasis under conditions of high shear stress.
| Malignancy | Lymphoma MGUS Waldenströms macroglobulinaemia Chronic Lymphocytic Leukaemia Hairy Cell Leukaemia Essential Thrombocythaemia Polycythaemia Chronic Myeloid Leukaemia Wilm's tumour Lung and bladder adenocarcinoma |
| Autoimmune disorders | SLE Thyroid disorders Connective Tissue disorders Graft v Host Disease [GvHD] |
| Cardiovascular Disorders | Aortic Stenosis Ventricular Septal Defect [VSD] Mitral Valve prolapse Left ventricular assist devices |
| Drugs | Antibiotics - Griseofulvin, Ciprofloxacin Anticonvulants - Valproic Acid Plasma volume expanders - Hydroxyethyl Starch [HES] |
| Miscellaneous | Uraemia Gastrointestinal angiodysplasia |
2. Type 3 von Willebrands Disease [VWD]
In some patients with Type 3 VWD and a complete absence of von Willebrand Factor secondary to homozygous null mutations or complete gene deletions, infusion of a VWF-containing concentrate may be associated with the development of alloantibodies to the infused VWF and which results in its rapid clearance.
This may occur in 5-10% of patients with Type 3 VWD. A consistent feature of anti-VWF antibodies is their specificity for VWF and lack of activity against Factor VIII. Interactions with Factor VIII, however, are possible as a result of the bound antibody blocking the Factor VIII binding site on the VWF molecule.
Diagnosis
Assays for Detecting VWF Antibodies
Assays for detecting anti-VWF antibodies are not as well established as the assays for detecting antibodies to Factor VIII in patients who have Haemophilia A or in acquired Haemophilia A. Some patients with AVWS may have anti-VWF antibodies that decrease the half-life of infused VWF but do not inhibit its function. A small number of antibodies inhibit VWF function and can be demonstrated in 1:1 mixing studies with normal plasma using a VWF:RCo assay. However, most anti-VWF antibodies do not inhibit VWF function but bind to and accelerate the clearance of VWF.
There is no standard laboratory approach for the identification of anti-VWF antibodies. In general, the available assays are based on the principle of a mixing study to demonstrate the inhibition of the platelet-dependent function of VWF, although recommendations to evaluate VWF function more broadly (including collagen-binding and FVIII-binding) exist. The anti-VWF antibodies do not demonstrate time and/or temperature dependence and the assay is typically done at 37°C with an incubation time between 15 minutes and 120 minutes. The antibody titre is reported in Bethesda units [Bu]. Negative results from mixing studies do not necessarily exclude the presence of an anti-VWF antibody, because it may be directed against non-functional epitopes.
More recently, some laboratories have used an enzyme-linked immunosorbent assay (ELISA) approach and, although these assays appear highly sensitive, there is concern about the rate of false positivity. Centralised testing in an experienced laboratory familiar with both the screening ELISA and the functional mixing studies is of value.
von Willebrand Factor Inhibitors
Inhibitory antibodies directed against von Willebrand factor (VWF) in Type 3 von Willebrand disease (VWD) may be detected by mixing Patient and Normal Platelet Poor Plasma [NPP] and then performing a VWF activity assay, such as a Ristocetin cofactor assay or Collagen-binding assay. Many VWF inhibitors, however, are non-neutralising and VWF inhibitors in non-VWD patients are rarely be detected in a Bethesda-type assay.
1. History: The history is essential and in particular a history of other disorders/drugs that might be associated with the development of AVWS. It is also important to establish if there any family history suggestive of a bleeding disorder and a family pedigree should be constructed.
2. Pharmacokinetics [PK] Profiles: In individuals with Type 3 VWD, a poor VWF recovery and/or rapid clearance of VWF after the infusion of a VWF-containing concentrate should raise the possibility of an inhibitor. PK profiles should be performed regularly in patients with Type 3 VWD. Similarly PK profiles can be very useful in individuals with suspected AVWS.
3. VWFpp to VWF:Ag ratio
The presence of antibodies targeting VWF promotes its rapid clearance from the plasma.
In the acidic compartment of the Golgi apparatus the pre-propeptide of VWF is separated from the mature VWF protein generating the VWF propeptide [VWFpp] and the mature protein. Upon endothelial stimulation or damage, the VWFpp together with VWF are secreted into the circulation.
The VWFpp has a concentration in plasma of 1 µg/ml and a half-life [T½] of 2-3 hours whereas the mature VWF protein has a concentration in plasma of 10 µg/ml and a T½ of 8-12 hours. The plasma level of the VWFpp is normally proportionate to the level of VWF:Ag and measurements of the VWFpp can aid in the detection of the rapid clearance of VWF.
Accelerated plasma clearance of VWF:Ag occurs in some patients who have AVWS, in those who have certain Type 1 VWD variants, or in those who have Type 3 VWD and who have alloantibodies to VWF. In such cases this is associated with an increase in the ratio of VWFpp to VWF:Ag.
4. Screening for an Inhibitory Antibody.
i. Mixing Studies:
a.
In AVWS, neutralising autoantibodies against VWF are rarely identified by mixing tests which detect residual VWF functional activity. Therefore, such studies have a limited role in clinical practice.
b. In Type 3 VWD if mixing studies are undertaken to screen for an inhibitor, the antibodies do not demonstrate time and/or temperature dependence. The assay is performed at 37°C with an incubation time between 15 minutes and 120 minutes. The antibody titre is reported in Bethesda units [Bu]. Negative results from mixing studies may not exclude the presence of an anti-VWF antibody, because it may be directed against non-functional epitopes.
VWF inhibitor assays using mixing studies involve comparing the VWF:RCo activity of a mix of patient and Pooled Normal Plasma [PNP] with a mix of buffer and PNP. Dilutions of the patient plasma may be required depending upon the inhibitor titre.
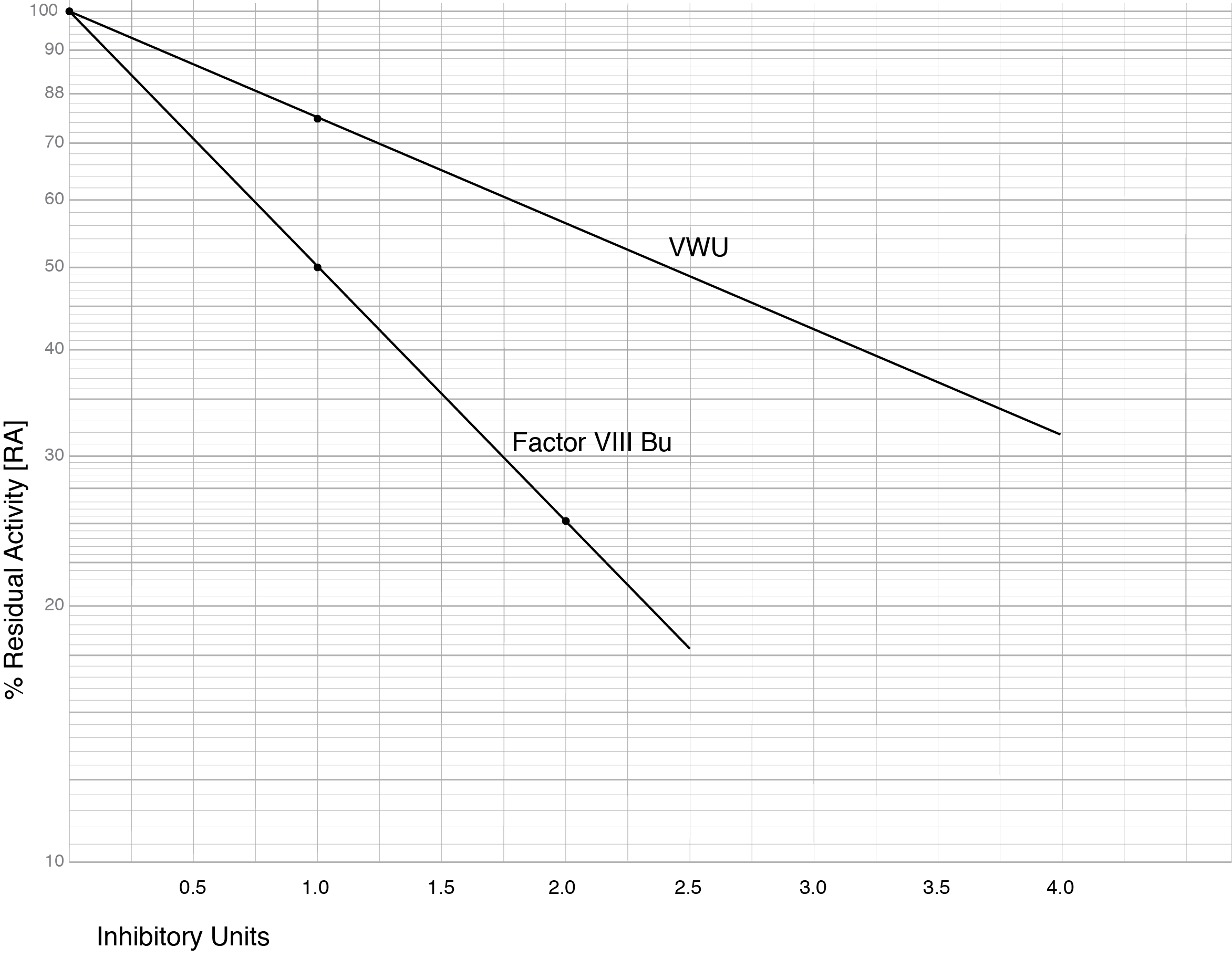
Miller 2021 - see References - reported VWF inhibitor levels, in a cohort of Type 3 VWD patients, to be much lower than VIII:C inhibitors measured at the same time. The VWF inhibitor screen was derived from measuring the VWF:RCo activity of a 1:1 mixture of Test Plasma and Pooled Normal Plasma [PNP] compared to a 1:1 mixture of PNP and buffer. The ratio of the two, multiplied by 100 - the % residual activity [%RA] - was then converted into an arbitrary unit termed VWU. 1 VWU representing the inhibition of 25% of the VWF:RCo activity in 1 mL of PNP or 75% residual activity and can also be derived from the equation:
VWU = [2-log %RA][0.125]-1 x Serial Dilution
where %RA is the percentage Residual Activity and Serial Dilution is any dilution of the patient sample that is required in cases of a high titre inhibitor.
The graph below shows a Log-Lin plot of a Factor VIII Bethesda Assay and a plot of the proposed VWU.
The Table below outlines [fictional] data from patients with Type 3 VWD and who have developed alloantibodies following infusion of a VWF concentrate. From this data and the graph above, the VWU titre can be calculated. In patients on treatment with a VWF concentrate and in whom the above mixing tests are planned, heat treatment of the patient plasma eradicates the VWF but does not affect the antibody levels. This is similar to screening for Factor VIII inhibitors in patients receiving treatment with a Factor VIII concentrate.
| Patient | VWF:RCo | VWF:RCO + PNP | Buffer + PNP | % Residual Activity [%RA] | VWU |
|---|---|---|---|---|---|
| 1 | <1 % | 28% | 51% | 55% | 2.0 units |
| 2 | <1 % | 0 1:2 dilution = 25 % |
52% 52% |
0% 48% |
5.2 units |
| 3 | <1 % | 48% | 47% | 100% | 0 units |
Patient 1: In this patient, the mix of patient plasma and PNP gave a VWF:RCo result of 28% and the %RA is therefore, 28/51 x 100 = 54.9%. From the graph this equates to a VWU titre of 2.0 units.
Using the formulae VWU = [2-log %RA][0.125]-1 then:
[(2-log 55)(0.125)-1 = (2 - 1.74) x (8) = 2.0 units.
There was no dilution of the patient plasma and therefore, no correction is required.
Patient 2: In this patient, the initial results using a mix of patient plasma and PNP gave a VWF:RCo result of 0% indicating a high titre inhibitor. Diluting the patient sample 1:2 allowed the calculation of the inhibitory titre. If a dilution is made the VWU titre derived from the graph must be multiplied by the dilution.
Using the formulae VWU = [2-log %RA][0.125]-1 then:
[(2-log 48)(0.125)-1 = (2 - 1.68) x (8) = 2.6 units. However, the sample was diluted 1:2 and therefore the actual inhibitor titre is 2.6 x 2 - 5.2 units.
Patient 3: In this patient, the RA is 100% and the VWU titre is, therefore, 0. Although this patient has an undetectable VWF:RCo result, no inhibitory antibody is detectable by mixing studies. This patient should be screened using other methodologies to exclude the presence of an inhibitor e.g. PK studies, VWFpp to VWF:Ag ratio, ELISA assays.
Using the formulae VWU = [2-log %RA][0.125]-1 then:
[(2-log 100)(0.125)-1 = (2 - 2) x (8) = 0 units.
In this patient, it is assumed that there is a reason to suspect an inhibitor such as an abnormal PK profile.
c. A different approach but again using mixing studies and the measurement of residual vWF:RCo from which the inhibitor titre is derived, is summarised below. See Kulkarni et al - References - for additional information.
Plasma samples from the patient are incubated at 56°C for 30 minutes to remove any residual VWF. As the VWF inhibitor titre is unknown, doubling dilutions in buffer of the plasma samples are made from 1:2 - 1:256. Each dilution is mixed in a ratio of 1:1 with Pooled Normal Plasma [PNP] and incubated at 37°C for 120 minutes. A reference plasma comprising a 1:1 mixture of PNP and buffer is also made and again incubated at 37°C for 120 minutes. At the end of the incubation period, the VWF:RCo concentration in each tube is measured and from which by comparison of the test value against the control value, the residual percentage VWF:RCo concentration can be derived. From a graph of the residual percentage VWF:RCo activity against inhibitory titre, the titre can be derived.
ii. ELISA Assays:
A number of ELISA assays have been reported for the detection of anti-VWF autoantibodies independently of their neutralising activity.
1. Siaka et al - 2003 reported a ELISA assay to detect the binding of IgG or IgM antibodies to VWF immobilised onto polystyrene plates See References for additional information.
2. Tiede et al 2008 used immobilised VWF is used to screen for the presence of anti-VWF antibodies. The plasma samples are diluted 1:10, 1:100 and 1:500 in Phosphate Buffered Saline [PBS]/Bovine Serum Albumin [BSA] and then added to the plates containing the immobilised VWF. The plates are incubated at room temperature for 60 minutes. The plates are washed to remove any non-adherent antibody and the bound anti-VWF antibody detected using an alkaline-phosphatase conjugated goat anti-human IgG or IgM antibody. p-nitorphoenyl-phospate is then added as a substrate and the change in absorbance [Optical Density - OD] measured at 405nm. The OD results are compared to normal plasma.
| Plasma Dilution | OD | Interpretation |
|---|---|---|
| 1:10 | <2 x Normal Plasma | Negative |
| 1:10 1:100 |
>2 x Normal Plasma <2 x Normal Plasma |
Weakly positive |
| 1:100 1:500 |
>2 x Normal Plasma <2 x Normal Plasma |
Positive |
| 1:500 | >2 x Normal Plasma | Strongly positive |
3. Franchi et al - 2014 - developed a two-step ELISA and confirmation assay using recombinant VWF for the detection of vWF antibodies in patients with AVWS. Plates are coated with rVWF. After washing with PBS-Tween 20, the wells in the plates are blocked with 2.5% bovine serum albumin in PBS. After washing, the plasma samples and positive/negative controls diluted 1/20 in PBS-1% BSA, are added to each well of the plate and incubated at room temperature for 120 minutes. IgG or IgM antibodies are then detected by the addition of sheep anti-human IgG or goat anti-human IgM conjugated to horseradish peroxidase. O-phenylenediamine is added as a substrate and the changes in OD are measured.
In the second step, samples positive in the ELISA assay undergo a confirmation assay with the addition of purified VWF or buffer, to exclude the 5% of false positive results obtained in the ELISA assay.
Click HERE to return to the top of the page.
