Introduction
The APTT in contrast to the Prothrombin Time [PT], measures the activity of the Intrinsic and Common pathways of coagulation. The division of the clotting cascade into the Intrinsic, Extrinsic and Common pathways has little in vivo validity but remains a useful concept for interpreting the results of laboratory investigations.
The term 'Thromboplastin' in this test refers to the formation of a complex formed from various plasma clotting factors which converts Prothrombin to Thrombin and the subsequent formation of the Fibrin clot.
The term 'Activated Partial Thromboplastin Time (APTT)' derives from the original form of the test (devised in 1953) in which only the Phospholipid concentration of the test was controlled (as opposed to the Phospholipid and the surface activator concentration) and the name 'Partial Thromboplastin' was applied at the time to Phospholipid preparations which accelerated clotting but did not correct the prolonged clotting times of the plasma derived from individuals with haemophilia. Essentially the term 'Partial' means Phospholipid is present but no Tissue Factor.
The APTT is also known as:
1. Kaolin Cephalin Clotting Time (KCCT) - do not confuse this with the Kaolin Clotting Time (KCT) which is a screening test for a Lupus Anticoagulant but which contains no Cephalin. Cephalin is a platelet phospholipid substitute.
2. Partial Thromboplastin Time with Kaolin (PTTK).
Historically, Kaolin was used as a surface activator. It binds directly to Factor XII [FXII] resulting in surface activation to XIIa. XIIa cleaves FXI to XIa but in the absence of calcium, activation of the subsequent factors does not occur. Kaolin is rarely used when the APTT is automated as its opacity makes the optical detection of the endpoint (i.e. the formation of a Fibrin clot) difficult. Commonly used activators for automated analysers include micronized Silica and Ellagic acid.
Cephalin is a phospholipid substitute that replaces platelet phospholipid in the test - the test uses platelet poor plasma [PPP] and so requires a source of phospholipid for coagulation to occur.
Principles
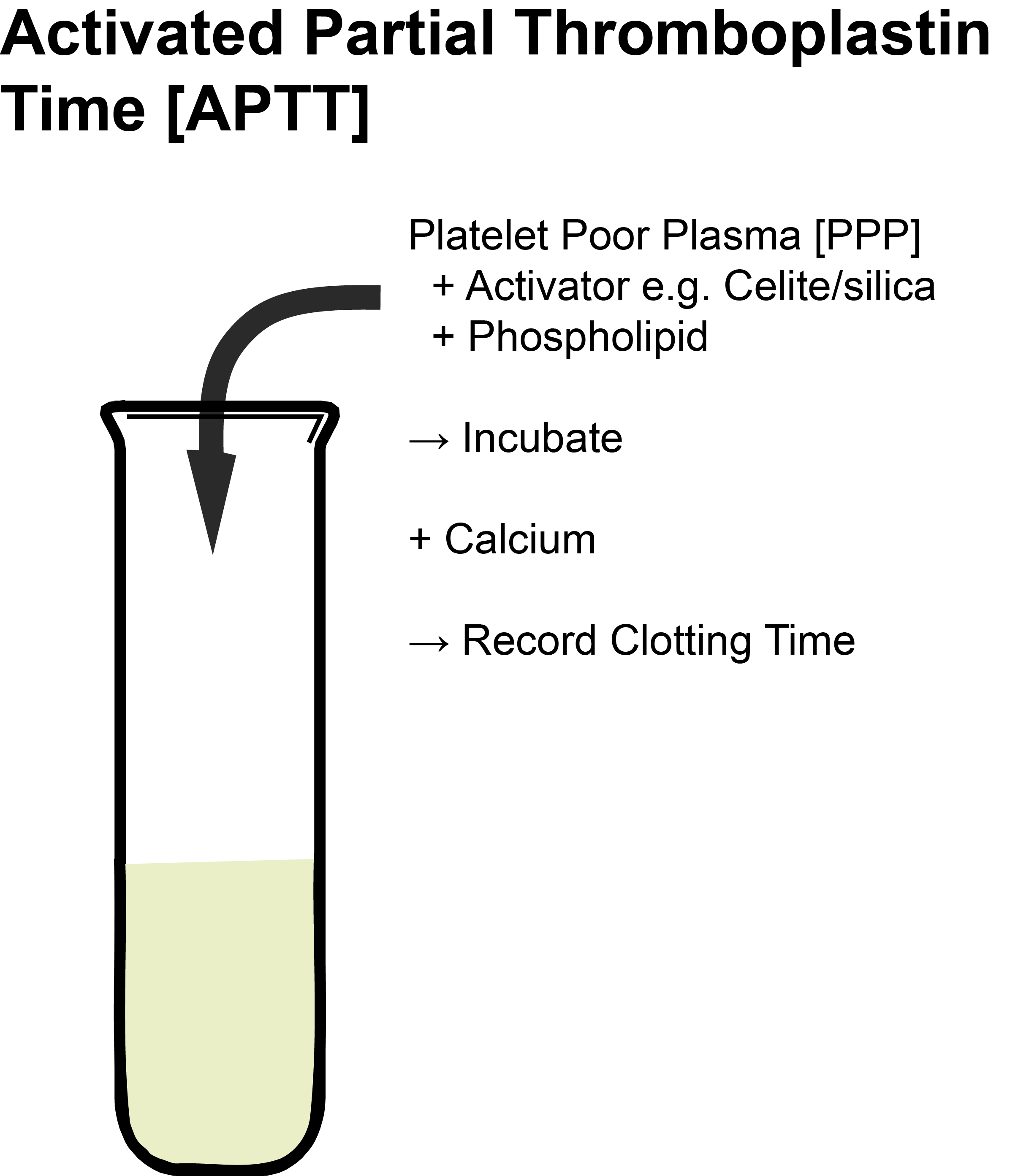
Platelet poor plasma [PPP] is incubated at 37°C then Phospholipid (Cephalin) and a contact activator (e.g. Kaolin, micronized Silica or Ellagic acid) are added. This leads to the conversion of Factor XI [FXI] to FXIa but the remainder of the pathway is not activated as no calcium is present. The addition of calcium (pre-warmed to 37°C) initiates clotting and the timer is started. The APTT is the time taken from the addition of calcium to the formation of a fibrin clot.
Most laboratories use an automated method for the APTT in which clot formation is deemed to have occurred when the optical density of the mixture has exceeded a certain threshold (clot formation makes the mixture more opaque and less light passes through).
The diagram below shows the clotting cascade. The factors that affect the APTT are shown in blue.
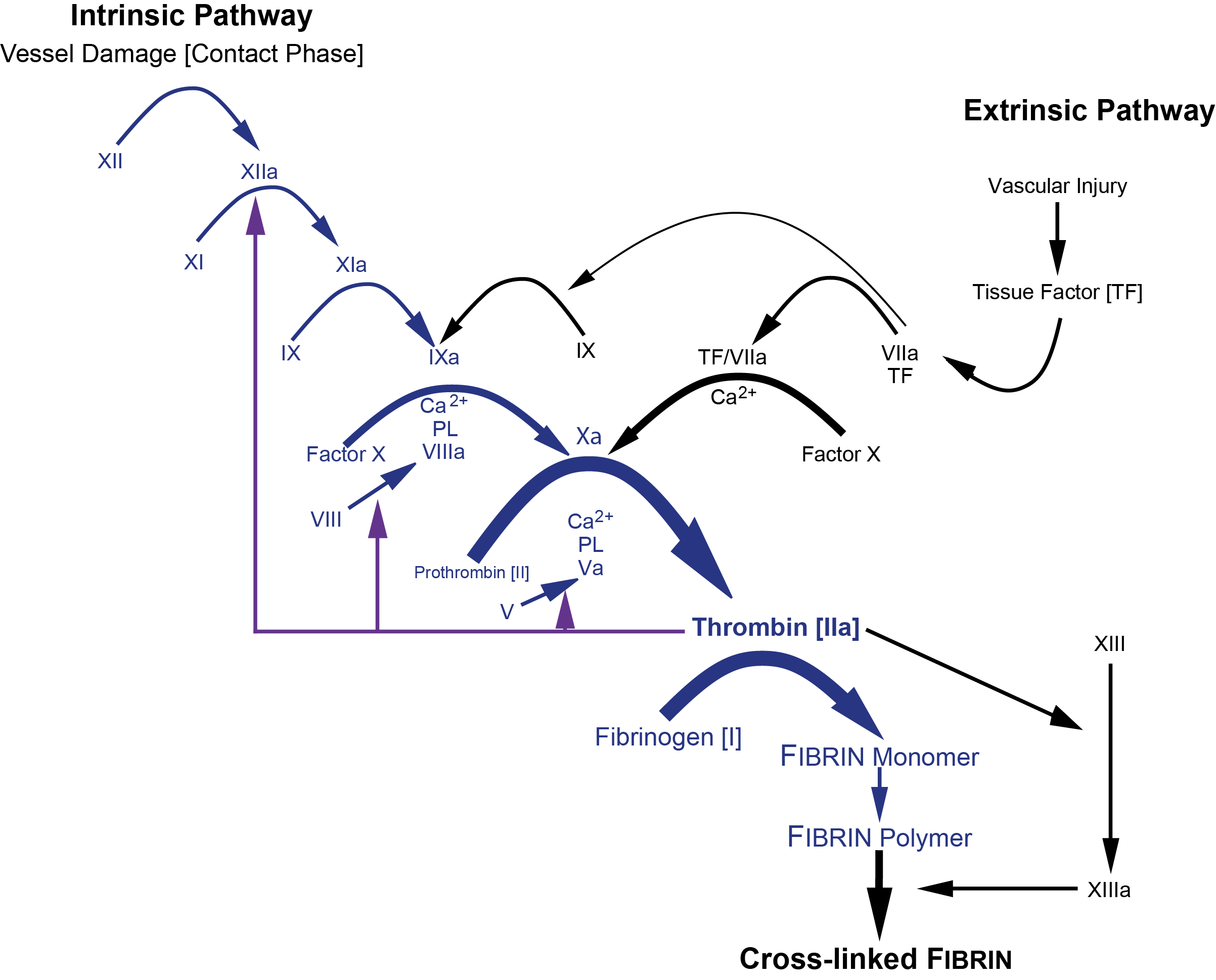
or more diagrammatically...
| Reagent | Explanation |
|---|---|
| Platelet poor plasma [PPP] | See Pre-analytical variables |
| Surface activator | Kaolin, Micronized silica, Celite, Ellagic acid |
| Phospholipid | e.g. Cephalin - to replace platelet phospholipid. Remember we are using Platelet Poor Plasma [PPP] in this test. The use of a commercial phospholipid substitute e.g. Cephalin means that the test can be standardised. |
| Calcium | Calcium is required in molar excess for coagulation to occur. Calcium is removed (by chelation) when blood is collected into sodium citrate and recalcification is necessary to allow coagulation to occur. |
Methodology
Platelet poor plasma [PPP] is incubated at 37°C with Phospholipid (Cephalin) and a contact activator (e.g. Kaolin) is added followed by the Calcium (all pre-warmed to 37°C).
The addition of Calcium initiates clotting and timing begins.
The APTT is the time taken for a Fibrin clot to form. Initial incubation times may vary from 2-10 minutes [- see comments].
Interpretation
The APTT is frequently performed as part of a series of screening tests that comprise the PT, APTT and often the Thrombin time and an estimation of the Fibrinogen concentration.
| Abnormality | Interpretation |
|---|---|
| Isolated Prolonged APTT | Deficiencies of either Factors XII, XI, IX & VIII. However, the APTT can be normal with mild deficiencies of these clotting factors. In general the deficient factor has to be less than 20-40% of normal before the APTT is prolonged [but see comments regarding APTT incubation times.] Contact factor deficiency e.g. pre-kallikrein deficiency, HMWK deficiency [In multiple clotting factor deficiencies the APTT becomes prolonged with less severe reductions in factor levels. Acquired clotting factor inhibitors - these are most commonly directed against FVIII and may occur as either autoantibodies [e.g. acquired Haemophilia A] or alloantibodies (in individuals with severe Haemophilia A following exposure to exogenous factor VIII e.g. the use of factor VIII concentrate to treat a bleed). Inhibitors against other clotting factors are rare but do occur e.g. Factor V. Lupus anticoagulant [LA] - a LA targets phospholipid and so can result in an isolated prolongation of the APTT. Although the PT requires phospholipid the concentration of PL in the PT reagents is high and so it frequently neutralises the LA and so the PT is not prolonged. |
| Prolonged APTT + Prolonged PT | Vitamin K deficiency Liver disease due to: - Malabsorption of vitamin K [a fat soluble vitamin] and which leads to decreased gamma carboxylation of the vitamin K dependent clotting factors - Decreased synthesis of clotting factors - An acquired dysfibrinogenemia due to changes in the sialic acid content of the fibrinogen. This is similar to the effect seen in the newborn infant - the so-called 'fetal fibrinogen.' Direct thrombin inhibitors including Hirudin, Argatroban and Dabigatran. DIC - due to the consumption of clotting factors. Massive blood transfusion leading to a dilutional coagulopathy. In patients receiving thrombolytic therapy, the APTT may be prolonged due to a reduction in fibrinogen levels. In multiple clotting factor deficiencies the APTT becomes prolonged with less severe reductions in factor levels. Combined deficiency of clotting factors e.g. Factors V and VIII. 'Common Pathway' clotting factor deficiencies - FX, FV, FII [Prothrombin] and Fibrinogen. |
| Increased APTT ± Prolonged PT |
Unfractionated heparin [UFH]: UFH significantly prolongs the APTT but the PT usually shows little prolongation. In cases of significant over anticoagulation with UFH the PT will be prolonged. Antiphospholipid antibodies [In some cases the PT can be prolonged although this is unusual due to the high PL concentration in PT reagents] Acquired clotting factor inhibitors e.g. FV, FX. |
| Prolonged PT ± Prolonged APTT | Warfarin - the APTT may be only prolonged by a few seconds in patients who are stably anticoagulated on Warfarin - but in patients who are overdosed the APTT may be significantly prolonged. |
| Short APTT | An acute phase response leading to high FVIII levels - see also Comment 8. Difficulties in the collection of samples leading to activation of coagulation within the collection tube. |
Reference Ranges
The clotting time for the APTT lies between 27-35 seconds. However, this varies widely between laboratories and is dependent upon a number of variables including whether the test is automated or manual, the type of activator and the incubation times employed in the test.
What Test Next?
Mixing studies: A mixing study in which patient plasma is mixed with normal plasma [ratio 1:1] may help to distinguish between a clotting factor deficiency and an inhibitor. If the mixture fails to correct the APTT within 3-4s this is strongly suggestive of:
- a coagulation factor inhibitor e.g. an acquired FVIII antibody
- an antiphospholipid antibody i.e. a lupus anticoagulant.
In practice, in many modern laboratories, mixing studies have been replaced by factor assays and a Lupus Anticoagulant screen as the process is almost fully automated.
The concept of mixing has been extensively investigated by Chang et al [see Refs] and the following formula has been used to demonstrate that the percentage correction of a prolonged APTT or PT in a 4:1 mix with control normal plasma [CNP] had an overall good specificity and sensitivity for detecting anticoagulants and factor deficiencies and is better than a 50:50 mix. The percentage correction is expressed in the following formula:

.... where PP is Patient Plasma and CNP is Control Normal Plasma.
The following table summarises the interpretation of the APTT in 4:1 mixing studies:
| Immediate % Correction | Incubated % Correction | Percentage Correction Suggests |
|---|---|---|
| ≥50% | >10% | Factor deficiency |
| <50% | >10% | Mild factor deficiency |
| ≥50% | ≤10% | Factor inhibitor |
| <50% | ≤10% | Lupus anticoagulant |
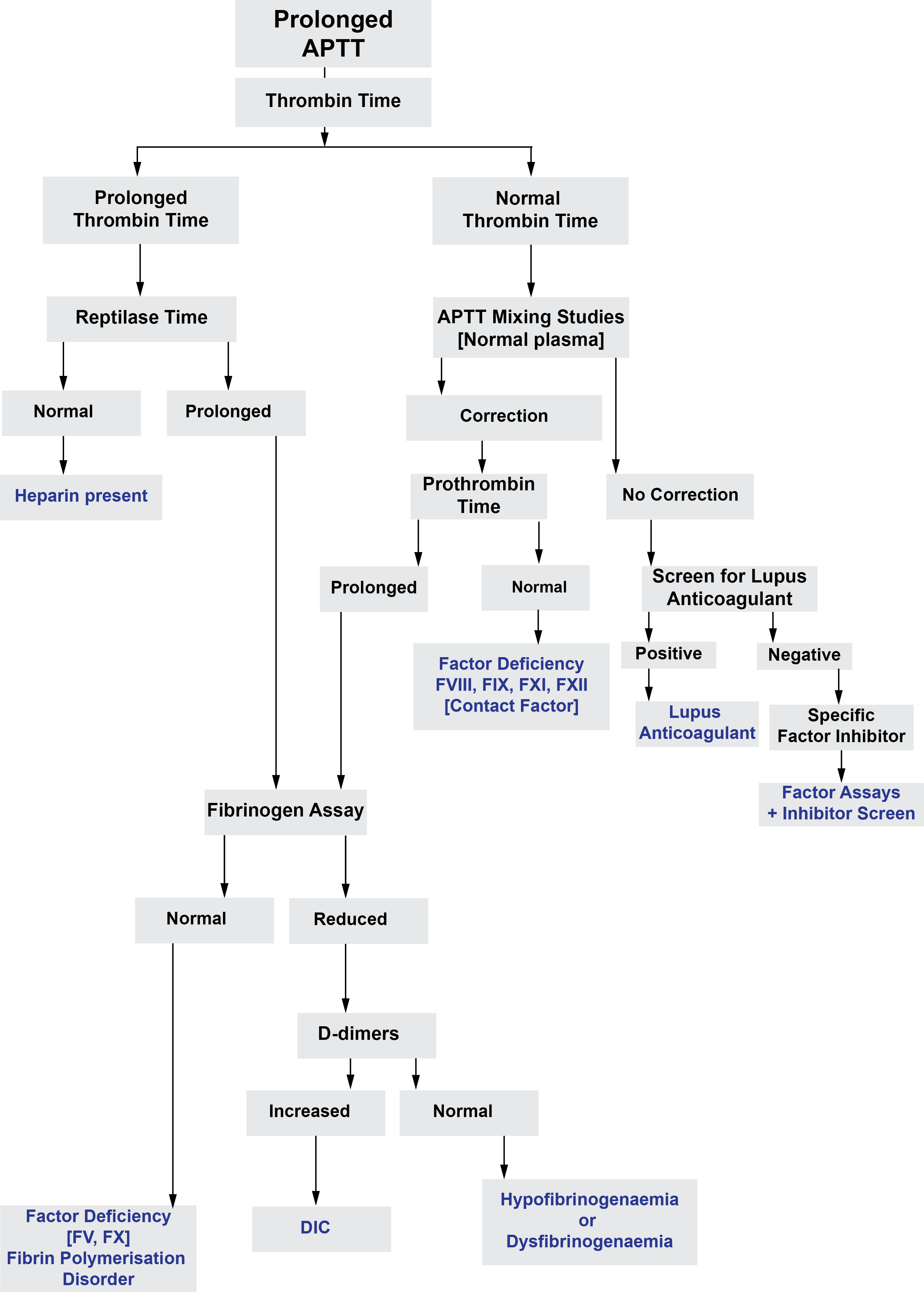
The figure below outlines the investigation of a prolonged APTT:
Click HERE to return to the top of the page.
