1. Global Fibrinolytic Capacity [GFC] Assay: Whole Blood
2. Global Fibrinolytic Capacity [GFC] Assay: Plasma
3. The Clot Formation and Lysis [CloFAL] Assay
4. Thromboelastography [ROTEM & TEG]
5. Overall Haemostatic Potential [OHP] Assay
6. Coagulation Inhibitor Potential [CIP] Assay
Fibrinolysis is the process by which Fibrin is removed from damaged blood vessels and is an integral part of the haemostatic system. Fibrinolysis is also important in tissue remodelling/repair after injury and in tumour metastasis. Disordered Fibrinolysis leading to bleeding, thrombosis and/or clinical outcomes in patients with various disorders have been reported.
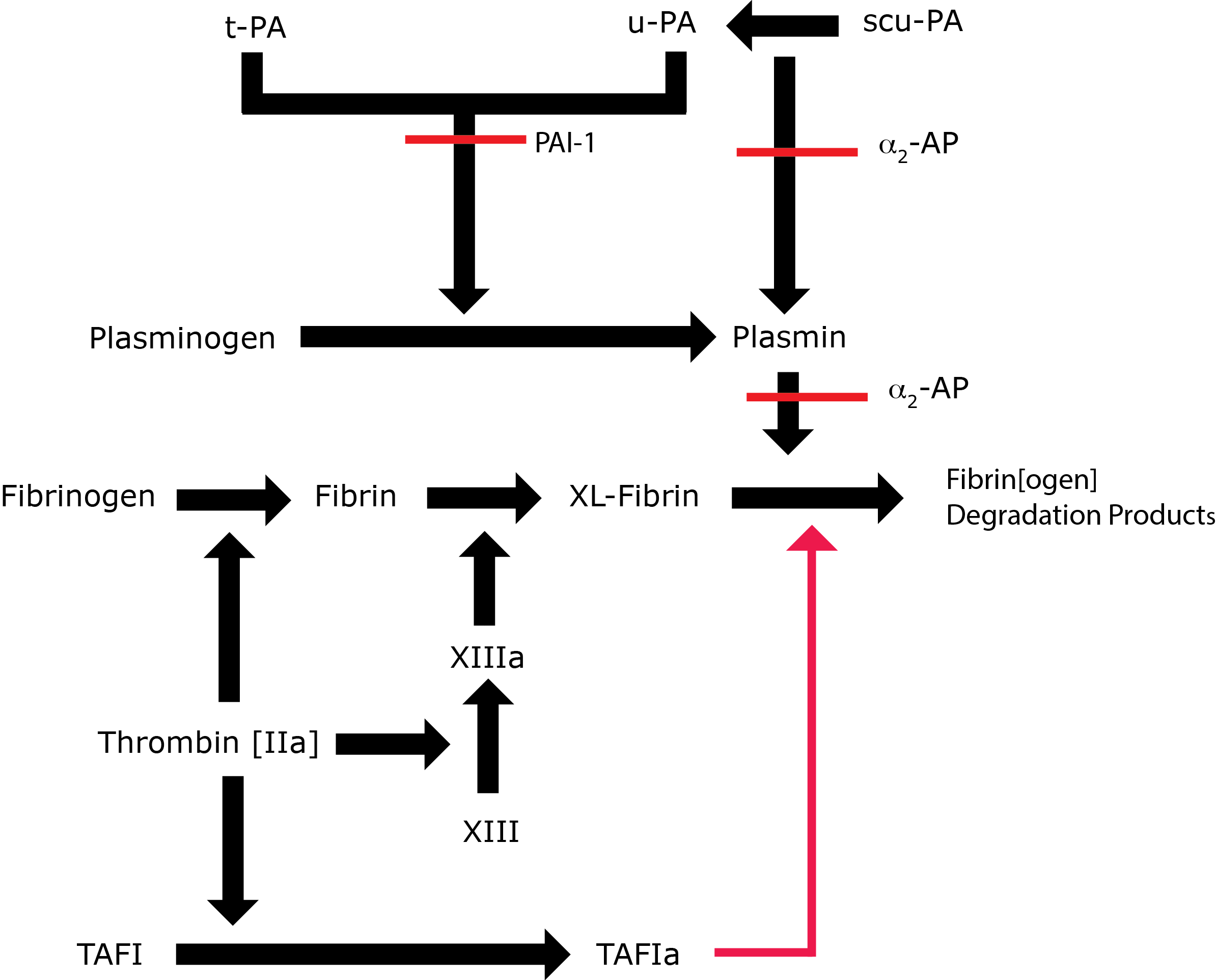
Key: t-PA - Tissue Plasminogen Activator; u-PA - Urokinase-type Plasminogen Activator; XL-Fibrin - cross-linked fibrin; TAFI - Thrombin Activatable Fibrinolytic Inhibitor; TAFIa - Activated Thrombin Activatable Fibrinolytic Inhibitor; α2-AP - Alpha-2-antiplasmin [Alpha2-Plasmin Inhibitor]; scu-PA - single chain Urokinase-type Plasminogen Activator. A red line indicates an inhibitory pathway.
1. Global Fibrinolytic Capacity [GFC] Assay: Whole Blood
Principles & Methodology
The Global Fibrinolytic Capacity [GPC] assay is a method for studying fibrinolysis in whole blood.
Whole blood is collected into a tube containing:
- Bovine serum albumin [BSA]
- Thrombin [6 NIH U/mL] ± Aprotinin [4000 KIU/mL]. Thrombin is included in the collection tube to initiate clotting and to prevent t-PA inactivation before the test has started. Aprotinin is included as a Plasmin inhibitor in one of the duplicate test samples.
The samples are incubated at
37°C for 3 hours. The clots are released from the tube wall and the samples centrifuged at 2000g for 20 minutes. Aliquots of the serum supernatant are collected, supplemented with Aprotinin and the Fibrin Degradation
Products (FnDP) measured using an ELISA assay. This assay system has been shown to be sensitive to t-PA and PAI-1 activity in addition to the Fibrinogen concentration.
Reference Ranges
The Global Fibrinolytic Capacity (GFC) is determined by subtracting the FnDP level obtained in the blood sample that was incubated in the presence of Aprotinin from the FnDP level obtained in the blood sample that was incubated in the absence of Aprotinin and the results expressed in µg/mL. The GFC determined in a group of 30 healthy subjects was
2.06 µg/mL [Range 0.13 - 13.6 µg/mL].
For further information see Rijken et al 2008.
2. Global Fibrinolytic Capacity [GFC] Assay: Plasma
Principles & Methodology
The assay is performed in a microcuvette at 37°C to which the plasma sample to be assayed is introduced with a fixed amount of Silica and t-PA. Calcium and Thrombin are then added to the sample to generate a Fibrin clot. Clot dissolution is recorded over time by measuring changes in light transmittance. The total assay time is ~60 minutes but this may be extended in the presence of hypofibrinolysis.
This method has been demonstrated to be sensitive to t-PA and PAI-1
activities, as well as the Fibrinogen level. Moreover, the assay is sensitive to fibrinolysis inhibition by TAFI, because the addition of a specific TAFIa inhibitor, PTCI [potato tuber carboxypeptidase inhibitor] significantly increases the Global Fibrinolytic Capacity or GFC.
The normal clot lysis time is 30-60 minutes with clot dissolution occurring within 45 minutes in most normal individuals. Hypofibrinolysis is >60 minutes. The GFC is slightly higher in females than in males and decreases progressively with age.
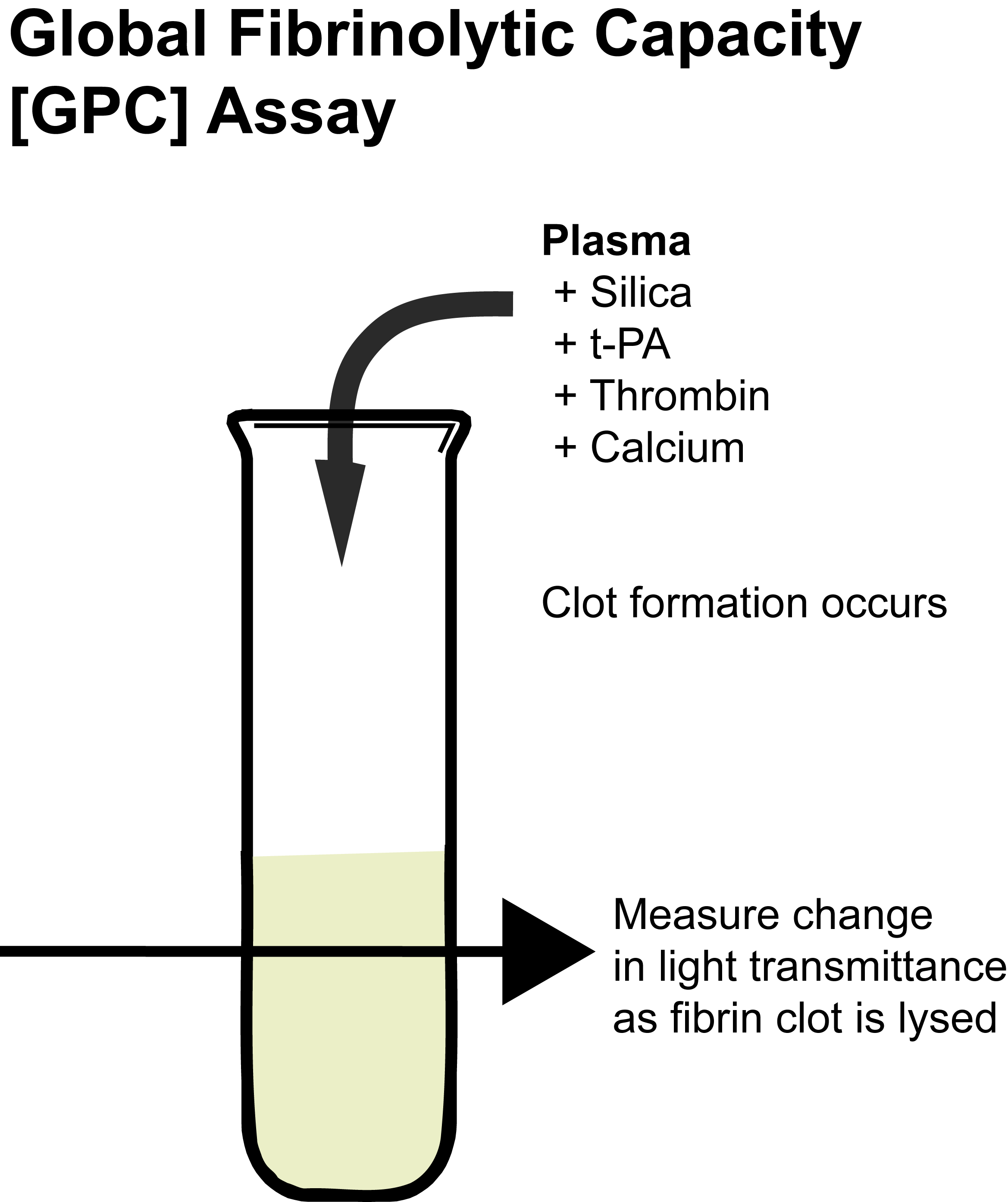
Reference Ranges
The GFC assay has a lysis time of 30 to 60 mins in normal individuals with a mean value of ~45 minutes. The GFC is slightly higher in females than males and increases with age. A significant correlation between GFC values and PAI-1 concentrations has been observed but in addition a significant correlation is also observed between t-PA antigen and TAFI highlighting the role that many factors play in the regulation of fibrinolysis.
For further in formation see Amiral et al 2018.
3. The Clot Formation and Lysis [CloFAL] Assay
The Clot Formation and Lysis [CloFAL] assay is a test that measures both coagulation and fibrinolysis and is sensitive to alterations in a number of the components that regulate coagulation and fibrinolysis. See Goldenberg et al 2005 for more information on this assay.
Principles & Methodology
1. An assay solution containing recombinant relipidated Tissue Factor [TF] to initiate coagulation and t-PA [450ng/ml - a low concentration to promote fibrinolysis] - is prepared in Tris-buffered saline [TBS] containing CaCl2.
An assay blank solution consisting of TBS alone [ i.e. it does not contain TF or t-PA] is also prepared.
2. 75µl of each citrated platelet-poor plasma sample is added to each of 4 wells in a 96-well microtitre place and then warmed to 37°C for 3 minutes.
3. 75µl of the TBS assay blank solution is added to the first well of each of the four plasma samples and 75µl of the reactant solution [pre-warmed to 37°C for 3 minutes] is added
to the remaining wells.
4. The plate is placed into a microplate spectrophotometer and absorbance measurements at
405 nm and 630 nm are undertaken at 45s intervals for 3hrs. The use of an assay blank allows for corrections to the absorbance date to be performed and removes potential problems due to icteric or lipaemic plasma samples. Each plasma sample is performed in triplicate.
A standard curve is generated and from which a number of variables are derived:
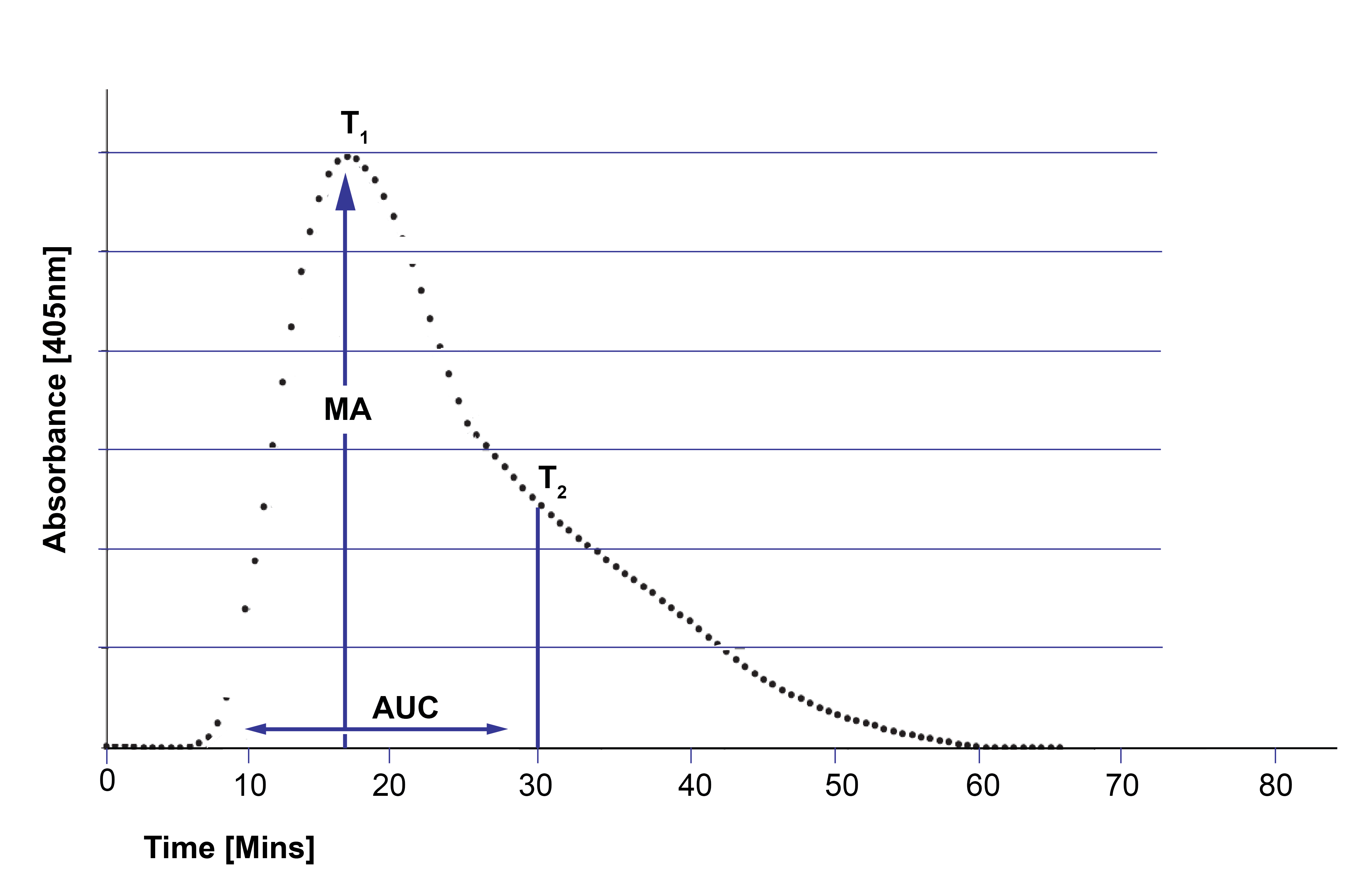
Maximum Absorbance [MA]:
T1 - Time to Maximum Absorbance [minutes]
T2 - the time at which the slope of decline in absorbance changes by +10 mOD/min
Area under the Curve [AUC] - measured from 0-30 minutes
Coagulation Index [CI]: =
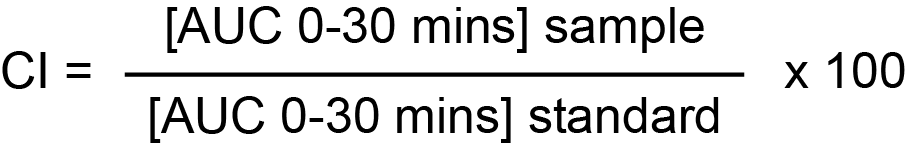
Fibrinolytic Index [FI]:
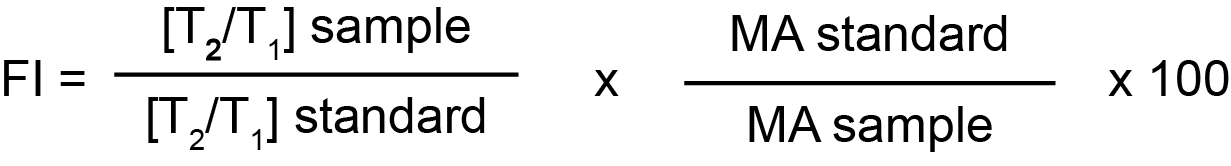
The median CloFAL CI in a group of normal health adults was 115% [range 53-93%]. The CloFAL CI varies in children and pregnant women.
For further information see: Goldenberg et al 2005.
4. Thromboelastography [TEG & ROTEM]
The TEG and ROTEM are generally used to assess coagulation but a number of parameters may be monitored to reflect endogenous fibrinolytic activity. For further information see the TEG and ROTEM pages.
5. Overall Haemostatic Potential [OHP] Assay
6. Coagulation Inhibitor Potential [CIP] Assay
The Overall Haemostatic Potential [OHP] assay is a global assay of Fibrin generation and Fibrinolysis and which has been evaluated in a number of clinical situations.
The Test involves the generation of a Fibrin Time Curve in which the generation of Fibrin and its subsequent lysis is measured by changes in absorbance at 405nm.
The Coagulation Inhibitor Potential (CIP) assay is a further development of the Overall Haemostatic Potential [OHP] assay in which a Pentasaccharide is added to the plasma sample to activate Antithrombin and so increases the sensitivity of the assay to functional abnormalities/deficiencies of Antithrombin and in addition Protac is included which activates Protein C to activated Protein C [APC]. In addition omitting t-PA allows more rapid testing recognising that abnormalities of Fibrinolysis are a rare cause of an inherited thrombophilia.
For more information on these assays, see Overall Haemostatic Potential [OHP] Assay and Coagulation Inhibitor Potential [CIP] Assay.
What Test Next?
Evidence of hyperfibrinolysis or hypofibrinolysis by global fibrinolytic assays requires additional investigations to establish the underlying cause. This may entail assays of individual factors involved in Fibrinolysis and/or sequence analysis of the relevant genes.
