Introduction
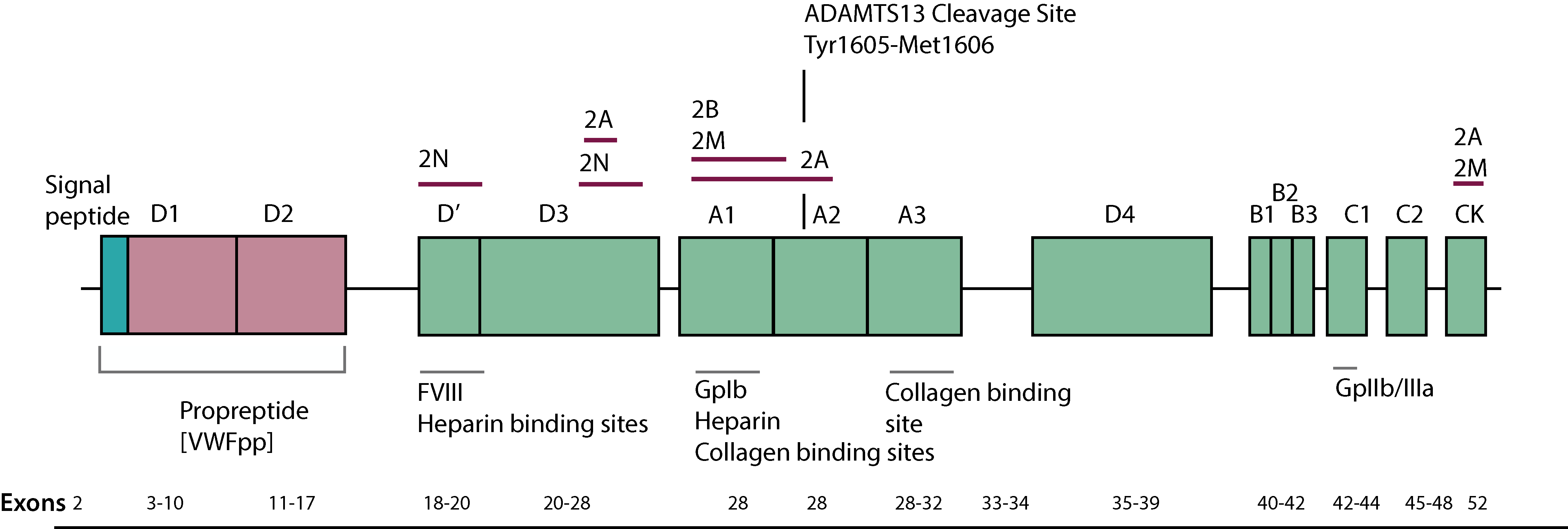
ADAMTS13 [the 13th member of ADintegrin-like And Metalloprotease with ThromboSpondin type 13 motifs] is a metalloprotease which limits platelet aggregation and microthrombi formation in the microcirculation by cleaving Von Willebrand Factor [VWF] between Tyrosine 1605-Methionine 1606 [Tyr1605-Met1606] located in the A2 domain, to generate a series of small molecular weight multimers.
A
deficiency of ADAMTS13 either because of an inherited mutation within the ADAMTS13 gene [ADAMTS13] or the development of an autoantibody, can lead to the potentially lethal syndrome of Thrombotic Thrombocytopenic Purpura [TTP].
A deficiency of ADAMTS13 leads to the accumulation of highly adhesive ultra large
VWF multimers [ULVWF] in the circulation and which result in the formation of microthrombi within small arterioles and the occlusion of these vessels. This, in addition to depleting platelets and resulting in thrombocytopenia, also leads to end-organ ischaemia and damage. Blood flow through the damaged/partially occluded vessels leads to red cell damage resulting in the characteristic red cell fragments seen on the blood film [schistocytes] and an intravascular haemolytic anaemia.
TTP is characterised by a pentad of signs/symptoms:
• Microangiopathic haemolytic anaemia [MAHA]
• Thrombocytopaenia
• Fever
• Renal impairment
• Neurological problems
However, the presence of thrombocytopenia and a MicroAngiopathic Haemolytic Anaemia [MAHA] without an alternative explanation, should raise the possibility of TTP or another Thrombotic MicroAngiopathy [TMA].
The gene for ADAMTS13 maps to the long arm of chromosome 9 at 9q34, spans 34kb of DNA and contains 29 exons. ADAMTS13 is synthesised primarily in the liver but there is some evidence that it can also be synthesised by endothelial cells.
Cleavage of Von Willebrand Factor [VWF] by ADAMTS13 does not normally occur because of inaccessibility of the Tyr1605-Met1606 peptide bond, which lies buried within the core β-sheet of the VWF. Shear forces, various denaturing reagents or the binding of platelets to VWF exposes the ADAMTS13 cleavage site within the A2 domain of VWF, allowing cleavage to take place. The VWF multimer pattern observed in plasma is due to the continued synthesis of VWF and its cleavage by ADAMTS13.
A large number of mutations within the ADAMTS13 gene have been reported in families with congenital TTP [Upshaw-Schülman syndrome]. In addition, there are a number of polymorphisms within the ADAMTS13 gene that may potentially affect its secretion and therefore, plasma levels.
Principles & Methods
A number of different methods and approaches have been developed to measure ADAMTS13 activity and to detect the present of any inhibitory antibodies. In general, measurement of ADAMTS13 activity is considered more clinically useful than assays which measure only the amount of ADAMTS13 present i.e. ADAMTS13 antigen.
As with all laboratory assays, laboratories providing tests for ADAMTS13 must include both internal and external quality control samples and participate in an external quality assurance programme. See - Quality Assurance - for more information.
| Assay | Comments |
|---|---|
| ADAMTS13 Antigen Assays | A number of immunoassays assays employing varying principles, exist for the measurement of ADAMTS13 antigen but they vary in their ability to detect full-length, mutant and truncated forms of ADAMTS13. ADAMTS13 antigen may be normal in TTP due to the presence of immune complexes, or reduced due to accelerated clearance. Assays of ADAMTS13 antigen are, therefore, generally unhelpful in the absence of a functional ADAMTS13 assay. In an ELISA assay for the measurement of ADAMTS13 antigen, the wells of a plate are coated with a monoclonal anti-ADAMTS13 antibody. Plasma samples or controls are added to the wells, incubated, washed and then an anti-ADAMTS13 antibody conjugated to Horse Radish Peroxidase [HRP] is then added to the wells of the microtitre plate and which binds to the immobilised ADAMTS13. A substrate for the HRP, TMB [3,3',5,5' TetraMethylBenzidene] is then added and incubated. The colour change is proportional to the ADAMTS13 concentration. A reference curve is created using controls of known ADAMTS13 concentration and from which the ADAMTS13 antigenic concentration in the unknown plasma sample can be derived. Other ELISA assays for the measurement of ADAMTS13 antigen employ similar principles. The wells of a plate are coated with an anti-ADAMTS13 antibody. Plasma samples or controls are added to the wells, incubated and then washed. ADAMTS13 in the sample or control will bind to the immobilised antibody. A human ADAMTS13 antibody conjugated to Biotin is then added to the wells, incubated and the plates then washed. The conjugated antibody binds to the immobilised ADAMTS13 and this is detected by addition by the addition of a Streptavidin-HRP [Horse Radish Peroxidase] solution. The Streptavidin binds to the Biotin and after incubation and washing, it is detected by adding a substrate for HRP, TMB [3,3',5,5' TetraMethylBenzidene]. This results in a colour change that is detected by measurement of the absorbance at 450 nm. A standard curve is constructed using controls of known ADAMTS13 concentration and from which the concentration of ADAMTS13 in the unknown samples can be derived. TMB is a chromogen that yields a blue colour when oxidized with Hydrogen Peroxide [catalysed by HRP]. |
| ADAMTS13 Activity Assays | These assays involve the detection of cleavage of products either of a full-length VWF molecule or a VWF fragment that encompasses the ADAMTS13 cleavage site [Tyrosine 1605-Methionine 1606 [Tyr1605-Met1606]]. A number of methods have been described. 1. SDS Agarose Gel electrophoresis and Western Blotting. In this assay purified VWF is incubated with plasma for 24 hours. Cleavage of the VWF by ADAMTS13 takes place leading to a reduction in multimer sizes. This reduction is visualised by agarose gel electrophoresis followed by Western blotting with a peroxidase-conjugated anti-VWF antibody. The concentration of ADAMTS13 activity in the test sample can be established by reference to a series of diluted normal plasma samples. However, analysis by this method is difficult with poor precision. 2. SDS-PAGE and Western Blotting. This assay is similar to the above assay but involves the visualisation of dimeric VWF fragments following SDS PAGE and Western Blotting. The assay is technically easier that SDS agarose gel electrophoresis and appears a very sensitive method for measuring ADAMTS13 activity levels. 3. Collagen Binding Assays. Normal plasma or purified VWF is incubated with the test plasma sample in the presence of BaCl2 and 1.5M urea which denatures the VW exposing the ADAMTS13 cleavage site. VWF is cleaved by ADAMTS13 and residual VWF is measured by its binding to collagen Type III. The bound VWF is quantitated using an ELISA assay with a conjugated anti-VWF antibody. 4. Ristocetin-Induced Aggregation. This is similar to the collagen-binding assay above but residual VWF is measured by Ristocetin-induced platelet aggregation using a platelet aggregometer. The above assays are rarely used and have been replaced by additional assays: 5. Fluorescence Resonance Energy Transfer [FRET] Assay The Technofluor® ADAMTS13 FRET assay requires two interacting proteins one of which is labelled with a donor fluorophore and the other is labelled with an acceptor fluorophore on a truncated synthetic 73 amino acid modified fragment of the A2 domain of VWF and which contains the cleavage site for ADAMTS13. The donor fluorophore is positioned close to the ADAMTS13 VWF cleavage site whilst the second fluorophore and which acts as a 'quencher', is located on the other side of the ADAMTS13 VWF cleavage site. Cleavage of the VWF fragment containing the two fluorophores by ADAMTS13, uncouples the two fluorophores and so allows the emission of fluorescence by the donor fluorophore and which can then be measured. The degree of fluorescence is directly proportional to the ADAMTS13 activity 6. Chromogenic ELISA Activity Assay. In the Technozym® chromogenic ADAMTS13 activity assay, a recombinant 73 amino acid VWF fragment tagged with Gluthathione S-Transferase [GST ] - GST VWF73 - is immobilised onto an ELISA plate using an antibody specific to Gluthathione S-Transferase [GST]. The recombinant VWF fragment encodes the A2 domain of VWF and this includes the ADAMTS13 cleavage site at Tyr1605-Met1606. Plasma is added to the immobilised GST-VWF73 fragment, incubated and cleavage of the immobilised fragment occurs at the ADAMTS13 cleavage site. After washing, a Horseradish Peroxidase [HRP] labelled monoclonal antibody specific to the cleavage site, is added to each well and incubated. After washing, TetraMethylBenzidene [TMB], a substrate for the HRP is added to each well and incubated. A stop solution is then added and the colour change which is proportional to the ADAMTS13 activity in the plasma samples, is determined by measuring the absorbance at 450nm. The activity assay kit includes calibrators which are used to create a calibration curve and from which the ADAMTS13 activity in the test samples can be established. 7. There are additional ELISA-based assays for determining ADAMTS13 activity - see references. |
| Anti-ADAMTS13 Autoantibody [Inhibitor] Assays | Two types of anti-ADAMTS13 antibodies have been reported in patients with acquired TTP: 1. Neutralising antibodies [~2/3 of cases] that inhibit the action of ADAMTS13. These are classically detected by performing a 1:1 mix of test:normal plasma and then measuring ADAMTS13 activity. ADAMTS13 antibodies may bind to any domain of the protease but the cysteine-rich spacer domain is consistently involved in antibody reactivity in patients who develop acquired TTP. 2. Non-neutralising antibodies [~1/3 of cases] that bind to and accelerate the clearance of ADAMTS13 from the plasma. These can be detected by Western blotting but more conveniently using an ELISA assay. 3. A Chromogenic assay for the determination of antibodies directed against ADAMTS-13 is available [Technozym]. This uses recombinant ADAMTS13 [rADAMTS13] immobilised onto the wells of a microtitre plate. The plasma sample is added and any ADAMTS13 antibodies present will bind to the rADAMTS13. The plates are then washed and any bound anti-ADAMTS13 antibody is detected using an anti-human IgG antibody conjugated to Horse Radish Peroxidase [HRP]. After incubation a HRP substrate [TMB] is added, incubated and the reaction is stopped with Sulphuric acid and the OD measured at 450nm. The colour intensity is proportional to the anti-ADAMTS13 antibody concentration. 4. ELISA assays for the detection of anti-ADAMTS13 antibodies have been developed and which can detect both inhibitory and non-inhibitory antibodies. In some cases IgG antibodies directed against ADAMTS13 may be detected in individuals with other auto-immune disorders particularly if the levels of the antibodies are high. |
| Bethesda Assay for the detection of inhibitory antibodies in immune-mediated TTP | A Bethesda assay for quantifying inhibitory antibodies to ADAMTS13 has been reported and is very similar to the Bethesda assay used for quantifying Factor VIII [FVIII] inhibitors. The assay can only detect anti-ADAMTS13 antibodies that functionally inhibit ADAMTS13 and may fail to detect individuals with high titre anti-ADAMTS13 antibodies and low ADAMTS13 antigen levels identified by an ELISA assay. The assay involves the heat treatment of the test plasma sample to inactivate any ADAMTS13 that may be present. An equal volume of the heat treated plasma sample is mixed with pooled normal plasma [NPP] with an an ADAMTS13 level >70 U/dL. A control sample is prepared using NPP and buffer instead of a test plasma. After incubation, the residual ADAMTS13 activity is quantified as a percentage of the control sample. 1 Bethesda Unit is defined as the amount of inhibitor i.e. anti-ADAMTS13 antibody, that neutralizes 50% of the ADAMTS13 activity of the control sample. |
Interpretation
There are a number of causes of a low ADAMTS13 activity measured by the above techniques. In classical TTP due to the presence of an autoantibody that either accelerates clearance of ADAMTS13 or inhibits its activity, ADAMTS13 during the acute phase is low (<10 IU/dL) or undetectable.
Measurement of ADAMTS13 activity in patients with a history of classical TTP is important because low levels have been shown to be predictive of relapse. ADAMTS13 IgG antibody and ADAMTS13 antigen levels correlate with outcome in TTP with increased cardiac and neurological involvement and increased mortality.
| ADAMTS13 Activity [U/dL] | Interpretation |
| 60–130 U/dL |
Normal result |
| ≤10 U/dL | Suggestive of TTP. Repeat test to confirm result. If repeat test confirms a low ADAMTS13 activity then an inhibitor assay should be undertaken and in some cases, sequence analysis of the ADAMTS13 gene. |
| 11–20 U/dL |
This result largely excludes TTP, but some cases of TTP e.g. patients undergoing treatment, may have similar values. |
| 21–60 U/dL |
May be seen in: i. Patients with TTP on treatment ii. Patients with a secondary TMA who are critically ill. Assessment of VWF level and activity may also be useful, as elevated VWF/ADAMTS13 ratios may be associated with increased risk of thrombosis. |
Reference Ranges
The normal range for ADAMTS13 ranges widely depending upon the method by which the assay is performed. In general the normal range lies between 60-130 IU/dL derived from healthy individuals.
TTP is characterised by a severe deficiency (<10 U/dL) of
ADAMTS13.
What Test Next
Assays of ADAMTS13 are usually performed to establish or to exclude a diagnosis of TTP. If a low ADAMTS13 activity is found, studies should be undertaken to establish if an inhibitor is present and if not one should consider the possibility of congenital TTP [Upshaw-Schülman syndrome]. Not all cases of congenital TTP present in childhood. In suspected cases of congenital TTP, sequence analysis of the ADMTS13 gene should be performed.
Click HERE to return to the top of the page.
