Introduction
Fibrinogen defects may be quantitative (hypo- or hyper-fibrinogenaemia) or qualitative (dysfibrinogenaemia). Inherited dysfibrinogenaemia is rare but an acquired defect of Fibrinogen function is more common, especially in liver disease when the Fibrinogen molecule is excessively glycosylated impairing its activity. Fibrinogen levels may also be reduced in liver disease due to reduced synthesis. Elevated levels of Fibrin Degradation Products (FDPs) also impair the conversion of Fibrinogen to Fibrin.
Fibrinogen assays are an important part of the investigation of a bleeding tendency or an unexplained prolongation of the APTT or PT. Elevated Fibrinogen levels may correlate with an increased risk of thrombosis in epidemiological studies although the significance in individual patients is unclear.
An elevated level of Fibrinogen is an established marker for coronary artery disease, peripheral vascular disease and stroke.
The structure of Fibrinogen is shown below:
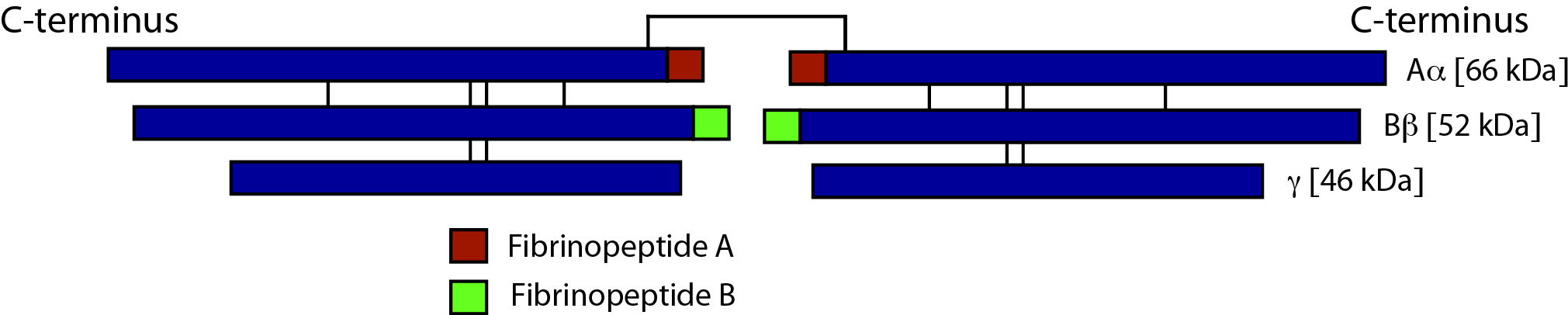
Fibrinogen is a hexamer and consists of three pairs of polypeptide chains: two Aα, two Bβ and two γ. These are linked together by 29 disulphide bonds in such a way that N-terminal regions of the 6-polypeptide chains meet to form a central E-domain. The C-terminal regions [Aα, Bβ and γ] form the D-domain and these are joined by α-helical ropes to the central E-domain to give the characteristic Fibrinogen structure. The polypeptide chains are encoded by three different genes - FGA [encoding the Aα chains], FGB [encoding the Bβ chains] and FGG encoding the γ chains. The Bβ-chain is encoded by 8 exons and generates a single transcript. The FGA and FGG genes encode two different transcripts:
i.
The major FGA transcript is encoded by five exons and comprises 97-99% of the circulating Fibrinogen. The minor FGA transcript is an extended Aα chain and arises from alternative splicing and is transcribed from six exons.
ii. The major FGG transcript is encoded by 10 exons and represents 85-92% of the total Fibrinogen. In the minor transcript, the 3′ end of
exon 9 is retained, replacing the four amino acids encoded by exon 10 by 20 C-terminal residues.
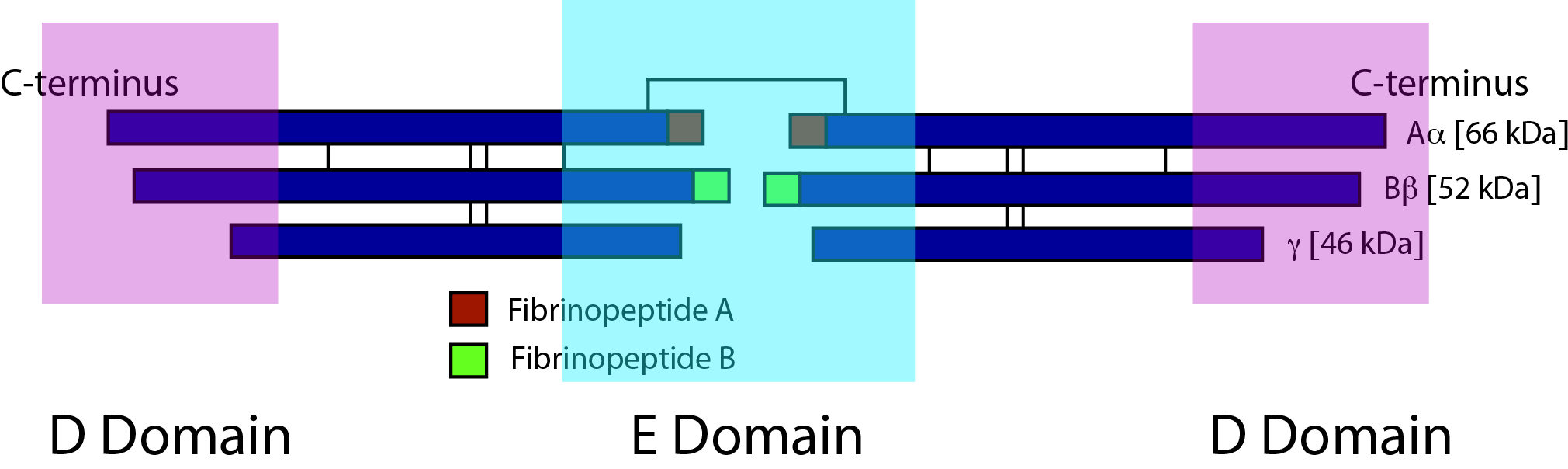
Activation of Fibrinogen by Thrombin [Factor IIa] cleaves two short peptides from the N-terminal regions of the Aα and Bβ chains - these peptides are known as Fibrinopeptide A [FpA] and Fibrinopeptide B [FpB] respectively. Removal of the N-terminal sequences from Aα and Bβ chains chains reveals new N-terminal sequences in the Aα and Bβ chains located within the E domain, known as 'knobs.' Thrombin cleaves FpA from the Fibrinogen E domain at Arg16-Gly17. These knobs can interact spontaneously with the D-dimer regions to form Fibrin polymers. Under the influence of Factor XIIIa, cross-linking of these Fibrin polymers then occurs to form cross-linked Fibrin polymers.
Principles
There are a number of assay for measuring Fibrinogen levels in plasma [see References] although in practice most laboratories use the Clauss method.
Fibrinogen Assays
| Assays | |
|---|---|
| Clauss Assay | A functional assay based upon the time for Fibrin clot formation |
| PT-derived Fibrinogen Assay | A functional assay based upon the time for Fibrin clot formation |
| Immunological Assay | An immunological method which measures Fibrinogen antigen rather than functional Fibrinogen |
| Gravimetric Assay | A method based upon clot weight [rarely preformed today] |
| TEG & ROTEM | The TEG and ROTEM machines can also be used to provide a qualitative indication of the Fibrinogen concentration. |
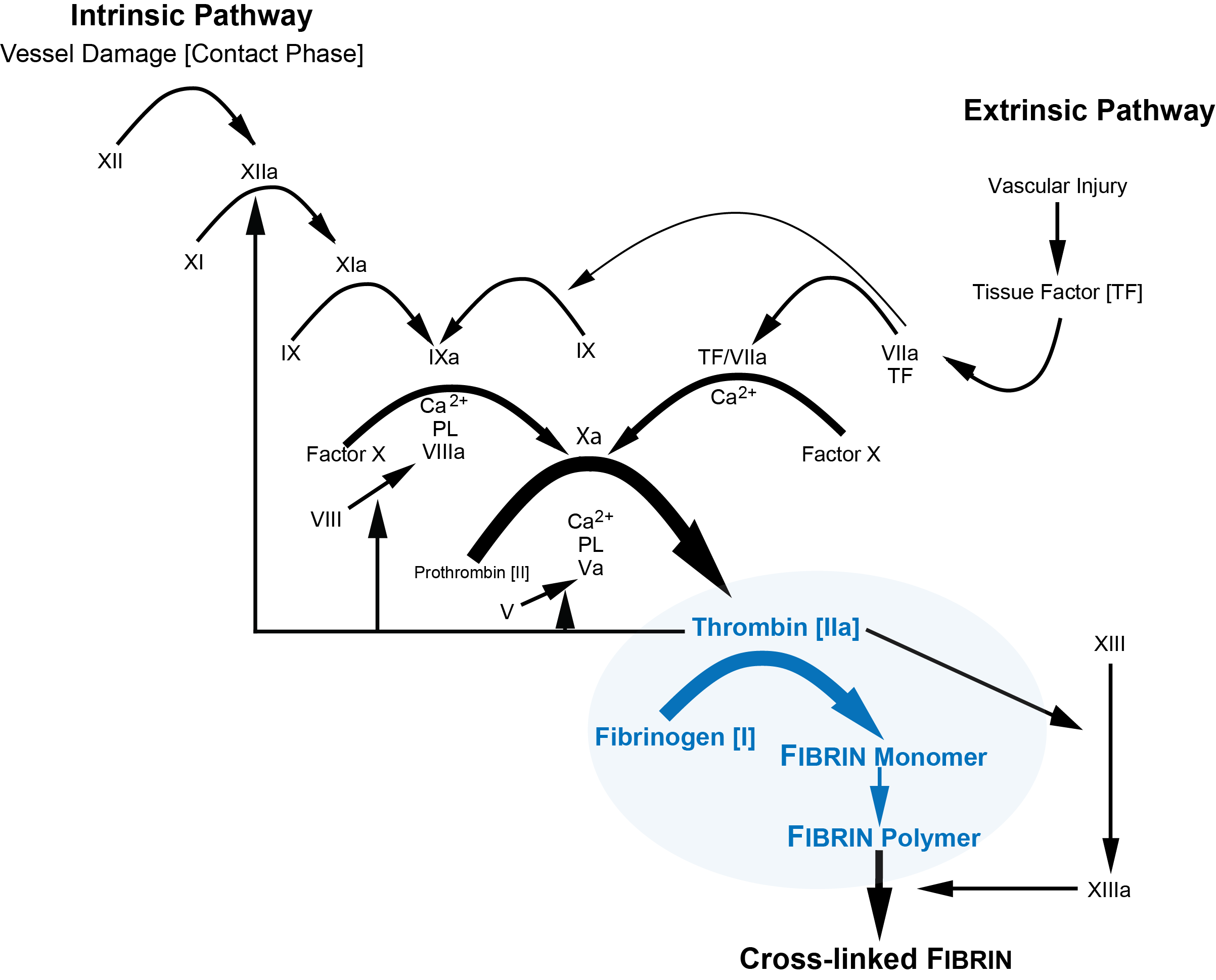
The diagram below illustrates the Clauss method for measuring Fibrinogen. Cross-linking of Fibrin by Factor XIIIa is not required for the Clauss Fibrinogen assay and so the Fibrinogen levels will be normal in severe Factor XIII deficiency.
Method
The methods for measuring Fibrinogen are summarised below:
| Assays | |
|---|---|
| Clauss Fibrinogen Assay | Diluted plasma is clotted with a high concentration of Thrombin [~100 U/mL]. 1. The test plasma is diluted (usually 1:10 but this may vary if the Fibrinogen concentration is very low or very high) to minimise the effect of 'inhibitory substances' within the plasma e.g. heparin, elevated levels of FDPs. The use of a high concentration of Thrombin (typically 100 U/ml) ensures that the clotting times are independent of Thrombin concentration over a wide range of Fibrinogen levels. 2. The test requires a reference plasma with a known Fibrinogen concentration and that has been calibrated against a known international reference standard. A calibration curve is constructed using this reference plasma by preparing a series of dilutions (1:5 –1:40) in buffer to give a range of Fibrinogen concentrations. The clotting time of each of these dilutions is established (using duplicate samples) and the results (clotting time(s)/Fibrinogen concentration (g/L) are plotted on Log-Log graph paper. The 1:10 concentration is considered to be 100% i.e. normal. There should be a linear correlation between clotting times in the region of 10-50s. 3. The test platelet poor diluted plasma (diluted 1:10 in buffer) is incubated at 37°C, Thrombin added (all pre-warmed to 37°C). The time taken for the clot to form is compared to the calibration curve and the Fibrinogen concentration deduced. Test samples in which the clotting times fall outwith the linear part of the calibration curve should be re-tested using different dilutions. Most laboratories use an automated method in which clot formation is deemed to have occurred when the optical density of the mixture has exceeded a certain threshold. |
| PT-derived Fibrinogen Assay | The PT is determined by optical density change for a range of plasma dilutions with known Fibrinogen levels. The optical change for each different Fibrinogen level is plotted as a calibration curve. A PT is performed on the patient's platelet poor plasma and the Fibrinogen derived from the change in optical density compared to the calibration curve. The derived Fibrinogen is a simple and inexpensive test and is widely used. However, the test can give misleading results in some disorders and is not recommended for routine laboratory use. |
| Immunological Assay | Assays based on enzyme linked immunoabsorbant assays (ELISA), radial immunodiffusion and electrophoresis are the most commonly employed. Immunological assays measure protein concentration rather than functional activity. They are of value in the investigation of congenital dysfibrinogenaemias where there is a discrepancy between functional activity and antigen level. |
| Gravimetric Assay | 1. Clot Weight Similar to the Clauss method: A Fibrinogen clot is formed by the addition of Thrombin and Calcium to diluted patient plasma. However, instead of using the time to clot formation to derive the Fibrinogen, the clot is compressed (to extrude plasma and unused reagents), washed, dried then weighed. This assay is technically difficult, time consuming and rarely performed in most modern laboratories. 2. Clottable protein Thrombin is added to plasma without Calcium and the clot formed is washed then dissolved in an alkaline reagent then spectrophotometry is performed (e.g. typically absorbance at 282nm). The clot is almost all Fibrin and so the measured protein concentration is taken as equivalent to the Fibrinogen concentration. |
| TEG & ROTEM | The TEG and ROTEM machines can also be used to provide a qualitative indication of Fibrinogen concentration - see Refs. |
The currently recommended choices of Fibrinogen assay for different clinical circumstances are shown below:
| Assays | |
|---|---|
| Investigation of an inherited bleeding disorder | Clauss [& Clottable Protein & Immunoassay] |
| Investigation of an acquired bleeding disorder | Clauss assay |
| Suspected dysfibrinogenaemia | Clauss & Clottable Protein & Immunoassay |
| Bleeding disorders affecting factors in addition to Fibrinogen (e.g. DIC) | Clauss assay |
| Thrombolytic therapy | Clauss assay |
| Hyperfibrinogenaemia | Clauss or Immunoassay |
Interpretation
| Fibrinogen Level | Interpretation |
|---|---|
| Fibrinogen levels are reduced in: | DIC due to the consumption of clotting factors Liver disease due to decreased synthesis. An abnormal Fibrinogen may be also be found in patients with liver disease due to an abnormal (increased) sialic acid content. Massive transfusion leading to a dilutional coagulopathy Inherited deficiencies e.g. Hypofibrinogenaemia, afibrinogenaemia and dysfibrinogenaemia [the latter is often associated with reduced Fibrinogen levels as well as activity] Following thrombolytic therapy In some patients following treatment with asparaginase Primary hyperfibrinolysis. This has been reported in individuals with carcinoma of the prostate and other cancers. |
| Fibrinogen levels are increased in: | Increasing age Female sex, pregnancy, oral contraception In post-menopausal women Acute phase reaction Disseminated malignancy [but may also be decreased if this is associated with DIC] |
Classification of Congenital Fibrinogen Disorders
Any individual with a suspected inherited Fibrinogen deficiency should have molecular studies undertaken to characterise a potential genetic abnormality.
| Type | Description |
|---|---|
| Quantitative | |
| 1. Afibrinogenaemia A. Afibrinogenaemia B. Afibrinogenaemia + thrombotic phenotype |
Asymptomatic or Haemorrhagic phenotype Thrombotic phenotype |
| 2. Hypofibrinogenaemia A. Severe B. Moderate C. Mild D. + Fibrinogen storage disease |
Functional Fibrinogen Levels < 0.5 g/L 0.5 - 0.9 g/L 1 g/L - lower limit of reference range + Evidence of Hepatocyte accumulation of Fibrin |
| Qualitative | |
| 3. Dysfibrinogenaemia A. Dysfibrinogenaemia B. Dysfibrinogenaemia + Thrombotic Phenotype |
Asymptomatic or bleeding phenotype. Thrombotic phenotype that does not fulfil the criteria for Type 3B. Dysfibrinogenaemia that is associated with a mutation that causes thrombosis. |
| 4. Hypo-dysfibrinogenaemia A. Severe B. Moderate C. Mild |
Fibrinogen Ag: < 0.5 g/L 0.5 - 0.9 g/L 1 g/L - lower limit of reference range |
Laboratory Tests in the Diagnosis of Congenital Fibrinogen Disorders
A number of tests can be undertaken to aid with the diagnosis of congenital Fibrinogen disorders. As always a personal and family history is essential and a family pedigree should be constructed in all cases.
| Test | Afibrinogenaemia | Hypofibrinogenaemia | Dysfibrinogenaemia | Hypo-dysfibrinogenaemia |
|---|---|---|---|---|
| PT | Prolonged +++ | Prolonged | Usually Prolonged | Prolonged |
| APTT | Prolonged +++ | Prolonged | Usually Prolonged | Prolonged |
| Thrombin Time | Prolonged +++ | Prolonged | Usually Prolonged | Prolonged |
| Reptilase Time | Prolonged +++ | Prolonged | Usually Prolonged | Prolonged |
| Functional Fibrinogen Assay | Undetectable | Decreased | Decreased | Decreased |
| Immunological Fibrinogen Assay | Undetectable | Decreased | Normal | Decreased |
| Functional:Immunological Fibrinogen Ratio | - | >0.7 | <0.7 | <0.7 |
Reference Ranges
The reference range for Fibrinogen is generally between 1.5-4.0g/L. Levels are normal or near normal at birth but the presence of a Fetal fibrinogen due to alterations in its Sialic Acid content can prolong the Thrombin Time.
What Test Next
The Fibrinogen level is usually interpreted in the light of other clotting tests such as the Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT).
i.
If the Clauss Fibrinogen assay is significantly reduced, both the APTT and PT will be prolonged. Although this indicates a hypofibrinogenaemia it does not exclude additional defects in the coagulation cascade such as may be found in Disseminated Intravascular Coagulation [DIC].
ii. Conversely, if the APTT and PT are prolonged but the clotting based Fibrinogen assay is normal, it suggests a defect higher up the clotting cascade and individual factor assays or a 50:50 mix with normal plasma may be helpful in establishing the cause.
iii. If the Clauss Fibrinogen assay suggests a reduced Fibrinogen level but there is no obvious reason for this and there is an appropriate clinical context (e.g. a family history of a bleeding diathesis, poor wound healing, umbilical stump bleeding) it may be useful to perform an immunological Fibrinogen assay to establish if the patient has a hypofibrinogenaemia or a hypo-dysfibrinogenaemia. If the Fibrinogen antigen test is not available, then a PT-derived Fibrinogen/Clauss ratio can be performed to aid with diagnosis.
In all cases of a suspected Fibrinogen deficiency, sequence analysis of the genes [FGA, FGB, FGG] encoding the Fibrinogen chains should be undertaken both to confirm the diagnosis but also to allow the investigation of at-risk family members and to provide informed genetic counselling.
Click HERE to return to the top of the page.
