Introduction
Protein C is a vitamin K-dependent serine protease of MW 62kDa and a key component of the natural anticoagulant pathway. Protein C consists of a 2-chain polypeptide - a light chain [155 amino acids] and a heavy chain [262 amino acids] linked by a disulphide bond.
The light chain consists of a Gla domain, with 9 glutamic acid residues that are gamma- carboxylated in the liver - a reaction which is Vitamin K dependent.. The GLA domain interacts with negatively charged phospholipid in the presence of calcium ions and is essential for the anticoagulant function of Activated Protein C [APC].
Protein C is converted by Thrombin [IIa] into its active form [Activated Protein C or APC) which, with its cofactor Protein S, degrades Factor Va and Factor VIIIa. Interestingly Factor V also serves as a cofactor for the inactivation of Factor VIIIa by APC.
The activation to PC to APC occurs relatively slowly in the presence of Thrombin alone but the rate of activation is increased significantly [20,000-fold] when thrombin is bound to the transmembrane protein Thrombomodulin [Tm] and Protein C is bound to the Endothelial Protein C receptor [EPCr]. Thrombin bound to Tm has no procoagulant activity but significant anticoagulant activity through the Protein C-Protein S pathway.
In addition to its anti-coagulant role, activated Protein C exhibits anti-inflammatory and anti-apoptotic activities. APC also binds to PAI-1 [Plasminogen Activator Inhibitor Type I] and inhibits its activity, so preventing inhibition of T-PA and thereby enhancing fibrinolysis.
Activated Protein C is inhibited by Protein C Inhibitor [PCI - historically known as PAI-3] - a member of the SERPIN family of SERine Protease INhibitors.
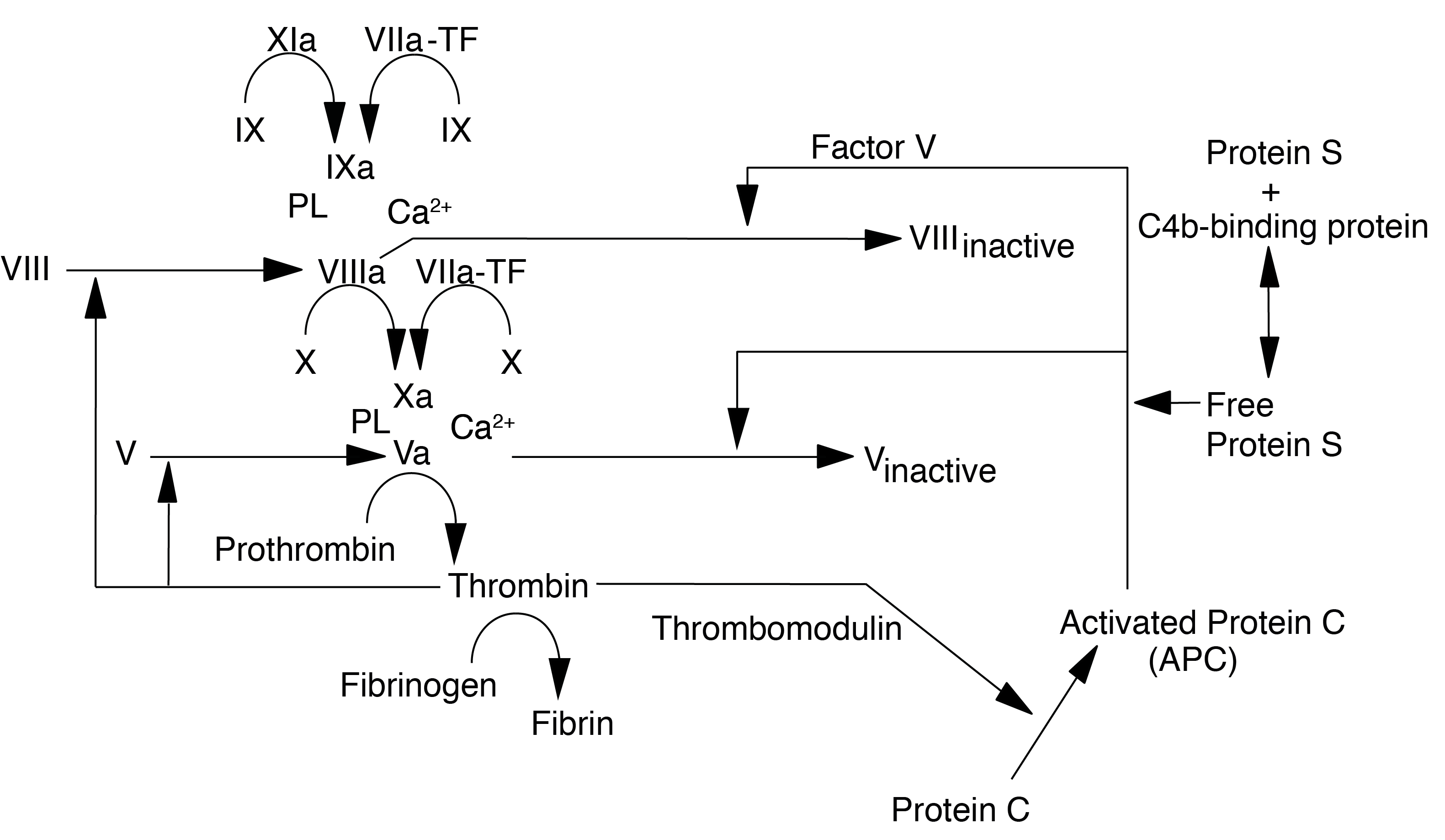
Protein C deficiency is inherited in an autosomal dominant manner and increases the risk of VTE to a magnitude depending on whether it is homozygous or heterozygous.
Homozygous Protein C deficiency is rare and presents in newborns with purpura fulminans (a form of disseminated intravascular coagulation characterised by extensive cutaneous haemorrhage and necrosis) which is rapidly fatal unless treated with Protein C replacement. In individuals with heterozygous Protein C deficiency, Warfarin may produce a similar phenomenon early in its use by reducing the level of Protein C which has a short half-life, before significant falls in the other vitamin K dependent procoagulants occurs. This, despite warfarin’s role as an anticoagulant, produces a procoagulant state characterised by thrombus formation in the small dermal vessels and extensive skin necrosis.
It is a rare phenomenon.
Activated Protein C resistance is the inability of Protein C to cleave factors Va and/or VIIIa. This may be hereditary or acquired but is usually due to abnormalities in the targets of Activated Protein C activity rather than to abnormalities of Protein C itself. The most common example of Activated Protein C resistance is due to the Factor V Leiden mutation.
Classification of Protein C Deficiency
Protein C deficiency has been classified into:
| Type I | Quantitative deficiencies. Accounts for 75-80% cases |
| Type II | Qualitative deficiencies. Accounts for 20-25% cases |
| Type IIa | Characterised by a low PC activity in a chromogenic assay; a low PC activity in the clotting based assay and a normal PC concentration |
| Type IIb | Characterised by a normal PC activity in a chromogenic assay; a low PC activity in the clotting based assay and a normal PC concentration |
Principles
Protein C may be measured by:
1. Immunological by means of an ELISA assay. This measures only the amount of Protein C present and NOT its functional activity.
2. A clot-based functional APTT assay - the time to clot formation after addition of a Protein C activator is determined and from this the amount of Protein C present can be determined.
3. A Chromogenic assay - Protein C is activated using (commonly) Protac™, an extract of the venom of Akistrodon contortrix contortrix and the concentration of Protein C is determined from the rate of colour change in the test sample due to cleavage of a chromogenic substrate.
4. A Thrombin generation based test has also been shown to detect Protein C deficiency.
Method
Commercial kits are readily available for Protein C Assays and these should use a reference Protein C standard calibrated against the current International Standard for Protein C.
1. Protein C ELISA Assays: Most ELISA assays use either monoclonal or polyclonal antibodies against Protein C.
2. Functional, Clotting-based Protein C Assays: These are based on either the PT or the APTT, although the APTT is more commonly used.
Patient platelet poor plasma is incubated at 37°C with phospholipid, a contact activator (e.g. Kaolin) and a Protein C activator (e.g. Protac). After incubation (typically 1-4 minutes) calcium is added to initiate clotting. The time taken to form a clot is measured. From this the Protein C level is determined from a reference curve.
The clotting time of the APTT [or PT] will be influenced by the amount of Va or VIIIa present in the reaction mixture and in turn this will be influenced by the activity of the Activated Protein C [APC]. APC is generated from the conversion of Protein C to activated Protein C by Protac. So if there is a reduction in circulating Protein C levels, then less APC will be generated, less Va and VIIIa will be inactivated and the clotting times will be shorter.
| Reagent | Interpretation |
|---|---|
| Platelet poor plasma | See pre-analytical variables |
| Surface activator | Kaolin, Micronized silica, Celite, Ellagic acid |
| Phospholipid | For example Cephalin - to replace platelet phospholipid |
| Protein C activator | Most commonly 'Protac' a snake venom |
| Calcium | Calcium is required in molar excess for coagulation to occur. Calcium is removed (by chelation) when blood is collected into sodium citrate |
3. Chromogenic
Protein C Assay: Patient platelet poor plasma is incubated at 37°C with the Protein C activator e.g. Protac. After incubation (typically 5 minutes) a chromogenic substrate for Activated Protein C is added. The change is optical density is measured and by comparison against a standard reference curve the Protein C level can be determined. Note that neither calcium, phospholipid nor a coagulation activator is necessary as the test plasma serves only as a source of Protein C and clot formation is unnecessary for this test. The chromogenic assay is not influenced by the presence of a lupus anticoagulant, Factor VIII level or the presence of the Factor V Leiden mutation.
Chromogenic assays will overestimate PC levels in patients taking vitamin K antagonists [VKA] e.g. warfarin.
PC levels do not reach adult levels until
puberty and age-appropriate
ranges must be used.
| Reagent | Interpretation |
|---|---|
| Platelet poor plasma | See pre-analytical variables |
| Protein C activator | Most commonly 'Protac' a snake venom |
| Chromogenic substrate | Generates a colour change which is proportional to the concentration of APC |
Interpretation
No single test for Protein C is 100% sensitive and specific for abnormalities.
1. An ELISA assay will measure Protein C immunological levels with very high sensitivity but will not detect functional defects. ELISA assays can show differences in specificity especially in clinical samples e.g. from patients on vitamin K antagonists. Protein C complexed to its inhibitor may be recognised by some ELISA assays but not others.
2. The
Chromogenic assay will detect low levels of Protein C with high sensitivity and will detect most functional defects but not all - for example, impaired phospholipid binding due to a mutation in the Gla domain will not be detected as the chromogenic assay is phospholipid independent.
An individual with a mutation in the GLA domain of the PROC gene will have normal a chromogenic Protein C assay. Similarly the chromogenic assay is not dependent upon the presence of Protein S.
The chromogenic assay can also be influenced by other serine proteases leading to an over-estimation of Protein C levels.
3. An
APTT-based functional Protein C assay can yield misleadingly low Protein C levels in the presence of the Factor V Leiden mutation and some other causes of activated Protein C resistance; elevated plasma factor VIII levels and in the presence of hyperlipidaemia. Falsely normal results may occur in the presence of lupus anticoagulants and in patients on direct thrombin inhibitors when using an APTT -based functional assay.
Clotting-based functional Protein C based assays are not recommended for routine use but can be of value in identifying Type IIb variants.
The following should be considered when interpreting results:
| Causes of apparently low Protein C levels | Causes of genuinely low Protein C levels |
|---|---|
| Factor V Leiden Other causes of Activated Protein C resistance Elevated plasma Factor VIII levels Hyperlipidaemia |
Hereditary: Heterozygous Protein C deficiency - seen in 0.2% of the population and in 3% of unselected patients with venous thromboembolism Homozygous Protein C deficiency - rare. |
| Acquired (more common than hereditary deficiencies): Acute phase reaction Disseminated intravascular coagulation [DIC] Liver disease L-Asparaginase therapy Vitamin K antagonists Prematurity Antiphospholipid antibodies Sickle cell disease |
Reference Ranges
The plasma concentration of Protein C in a healthy baby is in the region of 40 IU/dL. Levels may be very low in pre-term infants. Levels increase with age and reach levels of ~60 IU/dL but may not reach true adult values until after puberty. Normal Protein C levels in adults are in the region of 65–135 IU/dL.
What Test Next
APTT based tests may suffer from interference by Antiphospholipid antibodies and the patient should be screened for these.
Patients may be compound heterozygotes with a mixture of Type 1 and 2 defects and so it may be necessary to perform different functional assays as well as antigen measurement to confirm almost complete deficiency.
Homozygous Protein C deficiency is usually diagnosed in the neonate as levels are usually undetectable at presentation. However, the normal range for neonates is very wide and heterozygous deficiency may require repeat testing at 6 months to detect. If this is impractical, assays of other vitamin K-dependent coagulation Proteins for comparison or
measurement of parental levels of Protein C may be helpful.
Protein C determinations are usually performed as part of thrombophilia testing. Other tests include Protein S, genetic testing for FV Leiden and the G20210A Prothrombin gene mutations, homocysteine levels.
Acquired causes of a low Protein C should be tested for - levels of both Protein C and Protein S will be low in patients on vitamin K antagonists such as Warfarin.
Mutational analysis of the Protein C gene - PROC - should be undertaken in all cases of Protein C deficiency. This can be invaluable in genetic counselling based upon the natural history of similar mutations.
Click HERE to return to the top of the page.
