D-Dimer Assays
Introduction
Fibrinogen consists of three pairs of polypeptide chains: two Aα, Bβ and γ. These are linked together by 29 disulphide bonds in such a way that N-terminal regions of the 6-polypeptide chains meet to form a central E-domain. The C-terminal regions [Aα, Bβ and γ] form the D-domain and these are joined by α-helical ropes to the central E-domain to give the characteristic Fibrinogen structure.
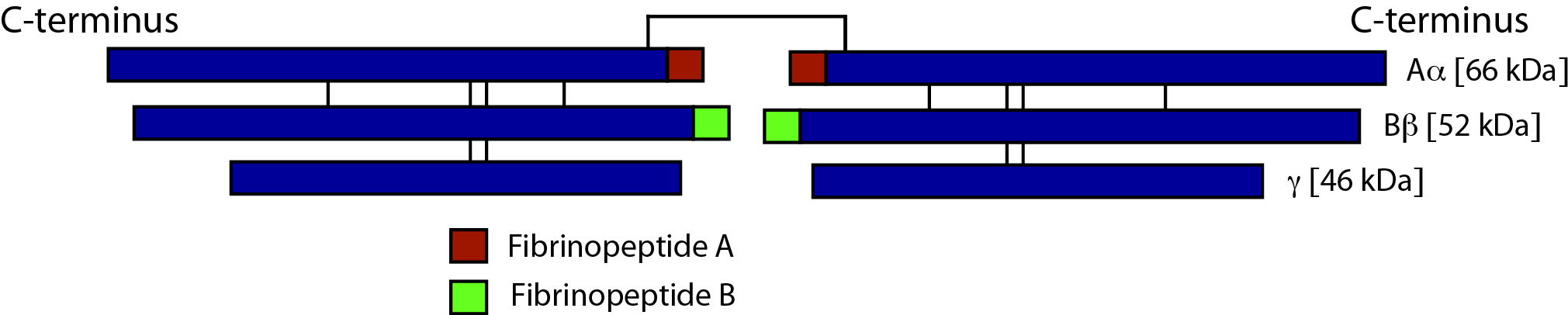
Activation of Fibrinogen by Thrombin [IIa] cleaves the two short peptides from the N-terminal regions of the Aα and Bβ chains - these peptides are known as Fibrinopeptide A [FpA - 16 amino acids] and Fibrinopeptide B [FpB - 14 amino acids] respectively. Removal of the N-terminal sequences from the Aα and Bβ chains chains reveals new N-terminal sequences in the Aα and Bβ chains located within the E domain, known as 'knobs.' These knobs can interact spontaneously with the D-domains to form fibrin polymers. Under the influence of factor XIIIa, cross-linking of these fibrin polymers [between glutamine and lysine amino acids] occurs to form cross-linked fibrin polymers.
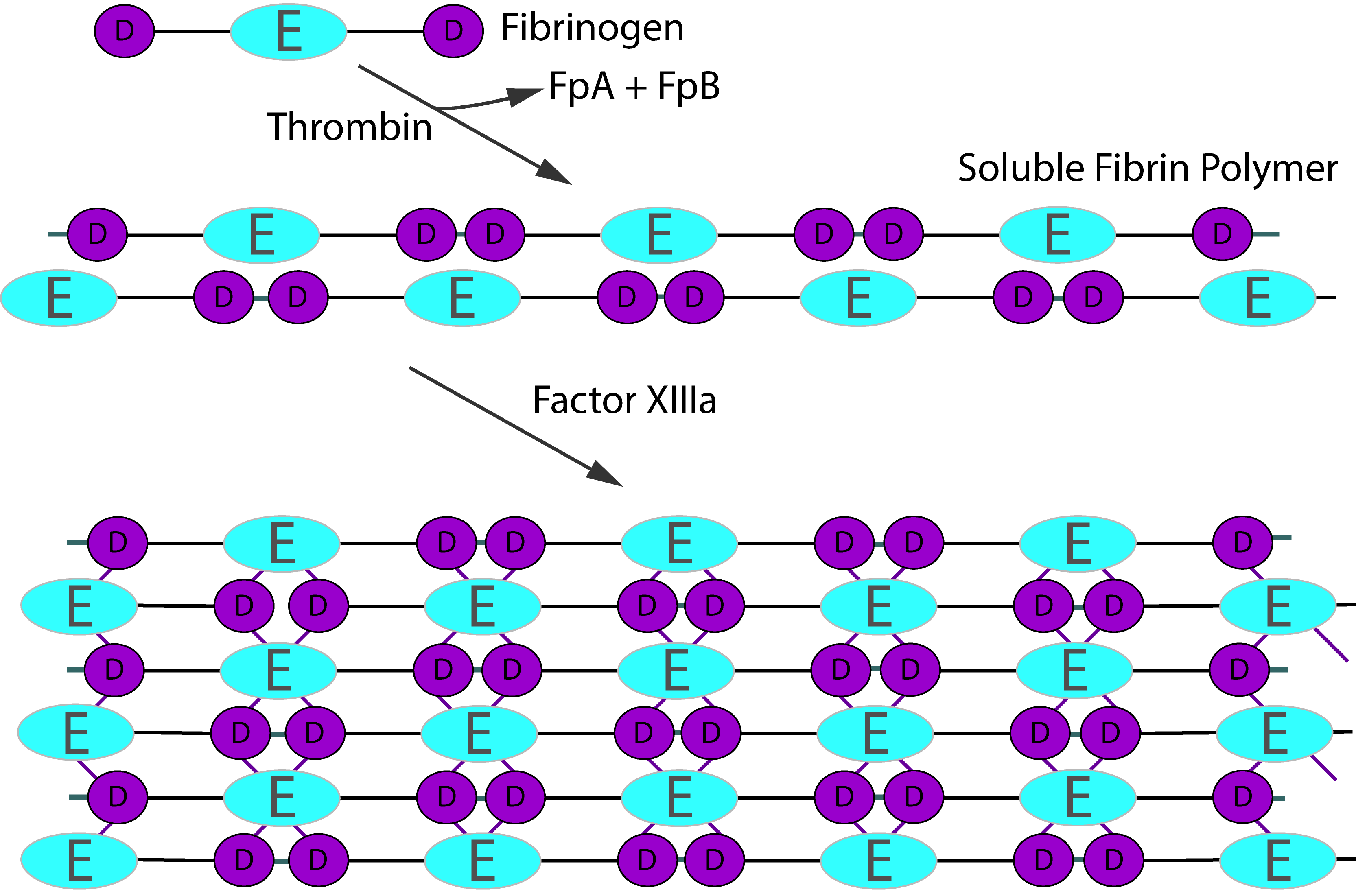
To recap the sequence is:
Fibrinogen → Fibrin monomer → Soluble fibrin polymer [non cross-linked] → Cross-linked fibrin polymer
Thrombin activation of Factor XIII is accelerated by the presence of non–cross-linked fibrin but is inhibited by fully cross-linked fibrin. This auto-regulation serves to help limit factor XIIIa activity to sites of fibrin clot formation. The principal sites of cross-linkage induced by FXIIIa are between the γ-domains in the D domains of adjacent fibrin monomers and between the carboxyl-terminal ends of the α chains of fibrin monomers.
D-Dimers
D-dimers are generated when cross-linked fibrin is degraded and so they are not generated if non-cross linked fibrin or Fibrinogen is broken down - they are, therefore, fundamentally different from Fibrin(ogen) Degradation Products [FDPs.] Fibrinogen Degradation Products [FDPs] consist of fragments X, D, Y and E.
1. Degradation of Fibrinogen and non-cross Linked Fibrin
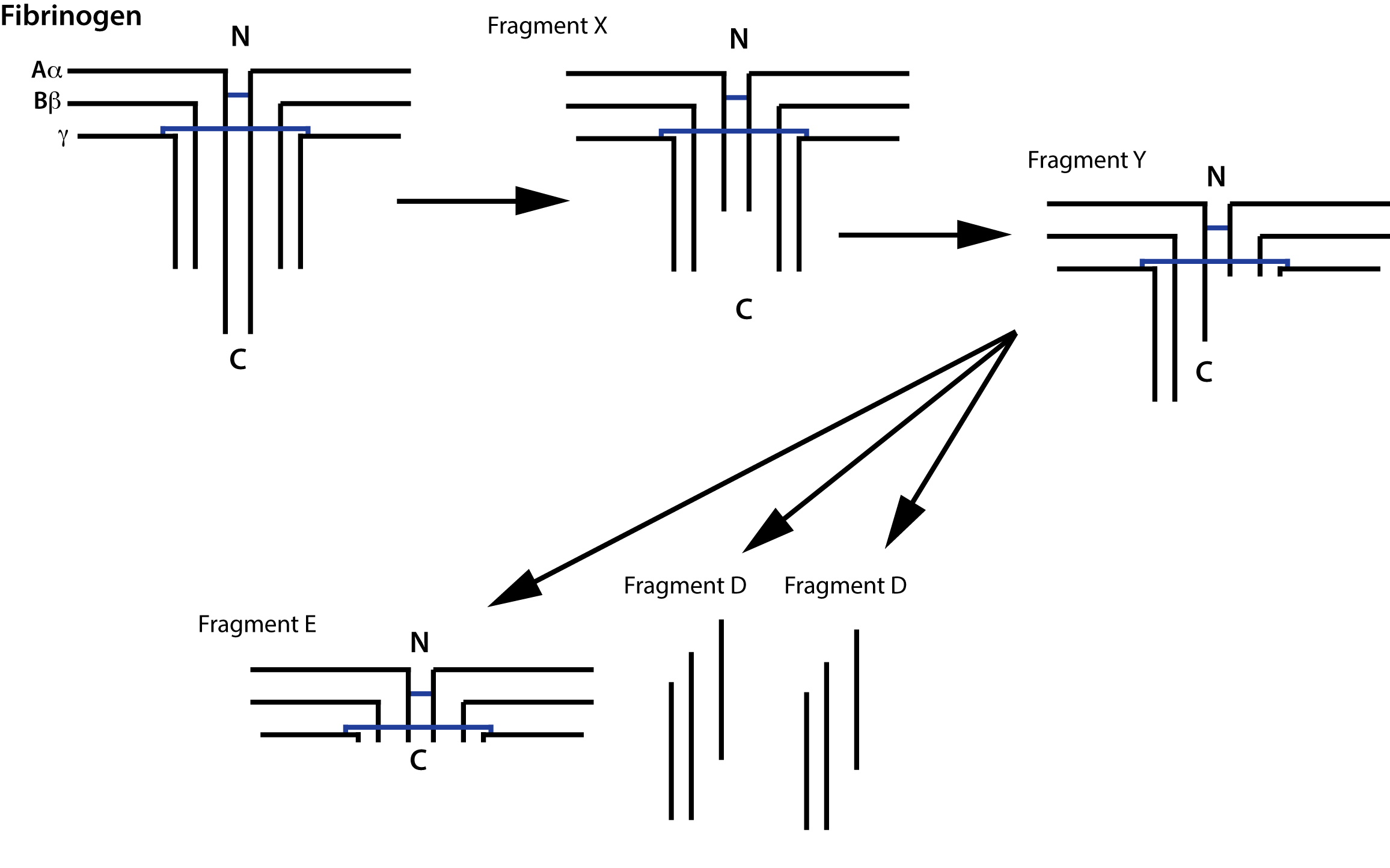
Fibrinogen is degraded by Plasmin generating a series of fragments [see above. Partial degradation of the γ chain gives rise to Fragment X and then further digestion of this fragment gives rise to Fragment Y. Digestion of Fragment Y gives rise to Fragment E and two D Fragments.
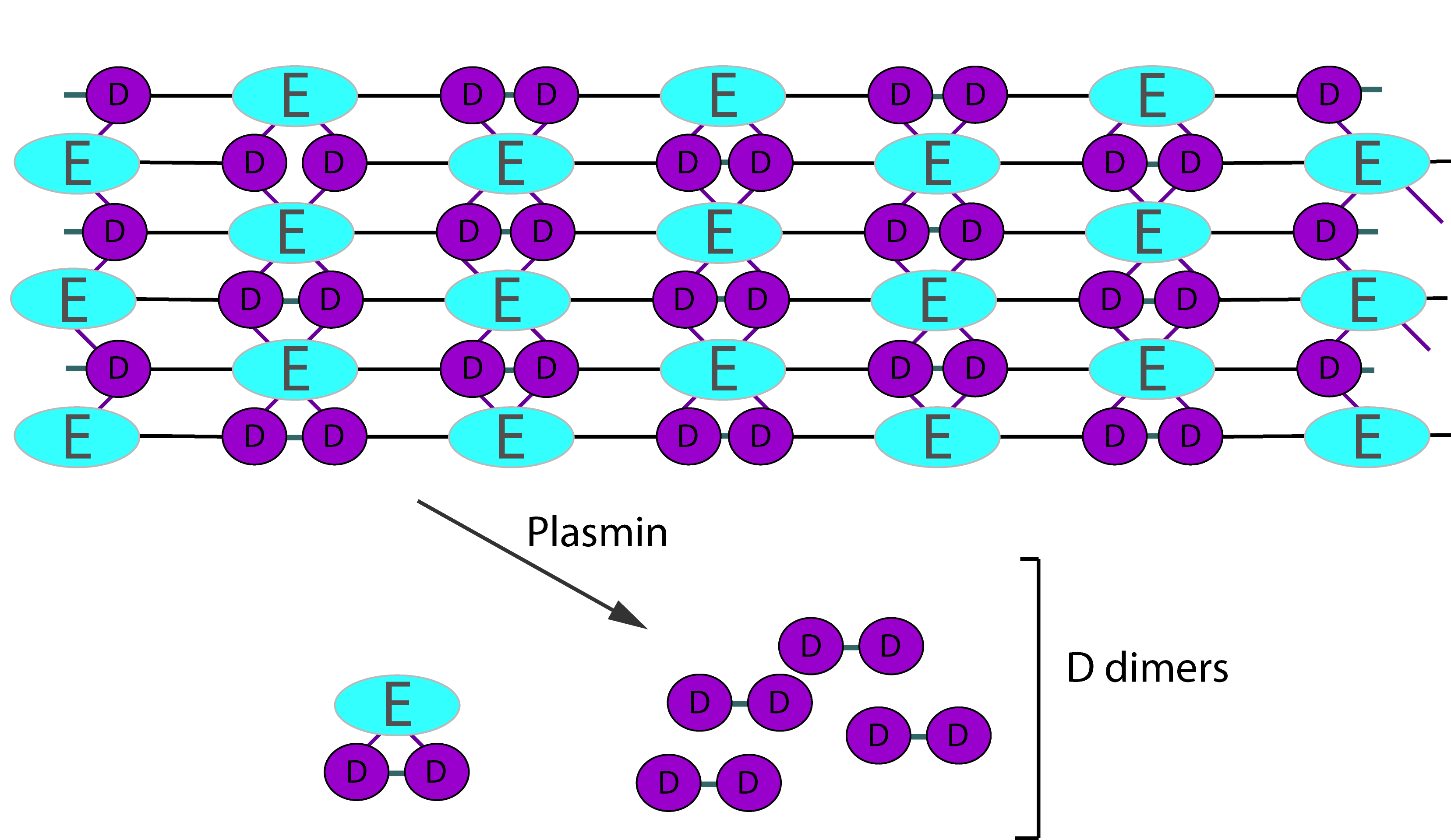
2. Degradation of Cross-Linked Fibrin
Digestion of cross-linked fibrin by Plasmin gives rise to a variety of fragments including D-dimers. These define the breakdown of cross-linked fibrin [i.e. fibrin that has been cross-linked by FXIIIa] as the cross-linking links D-fragments. Digestion by Plasmin cleaves cross-linked fibrin in a random order to generate a series of soluble fragments [so called 'X-oligomers'] of varying molecular weights and including D-dimers. In each of these breakdown steps, the γ–γ cross-links are retained and so D-dimers are present. Although the diagram below shows D-dimers in isolation, in practise they exists as a variety of D-Dimer containing oligomers e.g. EDD. The process is dynamic and more fragments are generated as the process proceeds. D-dimers create a neo-epitope and it is this that is detected by specific antibodies used in the various detection systems.
Principles & Method
Currently more than 30 different assays exist for measuring D-dimer but all use antibodies directed against the neo-epitope created by the formation of D-dimers. For a review of D-Dimer assays see References.
In general, 3 techniques are available to measure D-dimer although the sensitivities of these assays vary:
i. ELISA Assays using an antibody specific for D-dimer
ii. A whole blood agglutination assay that uses a bi-specific antibody conjugate with binding sites for both D-dimer and an antigen on the red cell. This assay uses whole blood.
iii. Latex particle agglutination assays that use bi-specific antibodies with binding sites for the latex particle and D-dimer.
It is important to remember that if a D-dimer assay is used as part of an algorithm for investigating patients for suspected venous thromboembolic disease, it must be validated for use in this clinical setting.
Interpretation
1. D-dimers are raised in DIC.
2. D-dimers are widely used to exclude a DVT and PE i.e. they have a high negative predictive value. However, D-dimers alone should not be used to exclude the diagnosis of a DVT in patients with a high pre-test clinical probability.
3. D-dimers are raised in a variety of clinical situations and so have a low positive predictive value for VTE. They can be raised in:
• Pregnancy
• HELLP
• Malignancy
• Infection including COVID-19
• DIC
• Vaso-occlusive sickle cell crisis
• Surgery/Trauma
• Burns
• Liver disease
• Snake bites
• Atrial fibrillation
• Renal failure
• Cardiac failure
• Venous thromboembolic disease [VTED]
• Aortic dissection
• Chronic inflammatory disorders
• Inflammatory bowel disease
• Thrombolytic therapy
4. D-dimer levels may fall in patients with a DVT treated with heparin.
Reference Ranges
It is important to remember that the reference ranges vary with the test used and there is currently no international D-dimer standard. Many laboratories use the manufacturers reference range. The best agreement between assays has been obtained using reference plasma preparations prepared from pools of clinical patient samples.
It is important to remember that not all D-dimer tests are suitable for the exclusion of VTED and
an assay with a high sensitivity used in well-designed studies comprising a patient population similar to that in which it will be used, should be employed.
