Introduction
A 1-stage APTT-based Factor assay is used for the measurement of Factors VIII, IX, XI and XII. It can also be used for assaying Factors X, V and II [Prothrombin] although a PT-based assay is more commonly used.
Factor XII can also be assayed using this technique as can Pre-Kallikrein [PRK] and High Molecular Weight Kininogen [HMWK] and although neither is associated with a bleeding tendency, they may result in significant prolongations of the APTT.
Principles
The assay of a clotting factor relies upon measuring the degree of correction of the APTT when plasma is added to a plasma sample specifically deficient in the factor to be measured. An outline of the principles of a 1-stage Factor VIII assay is shown below:
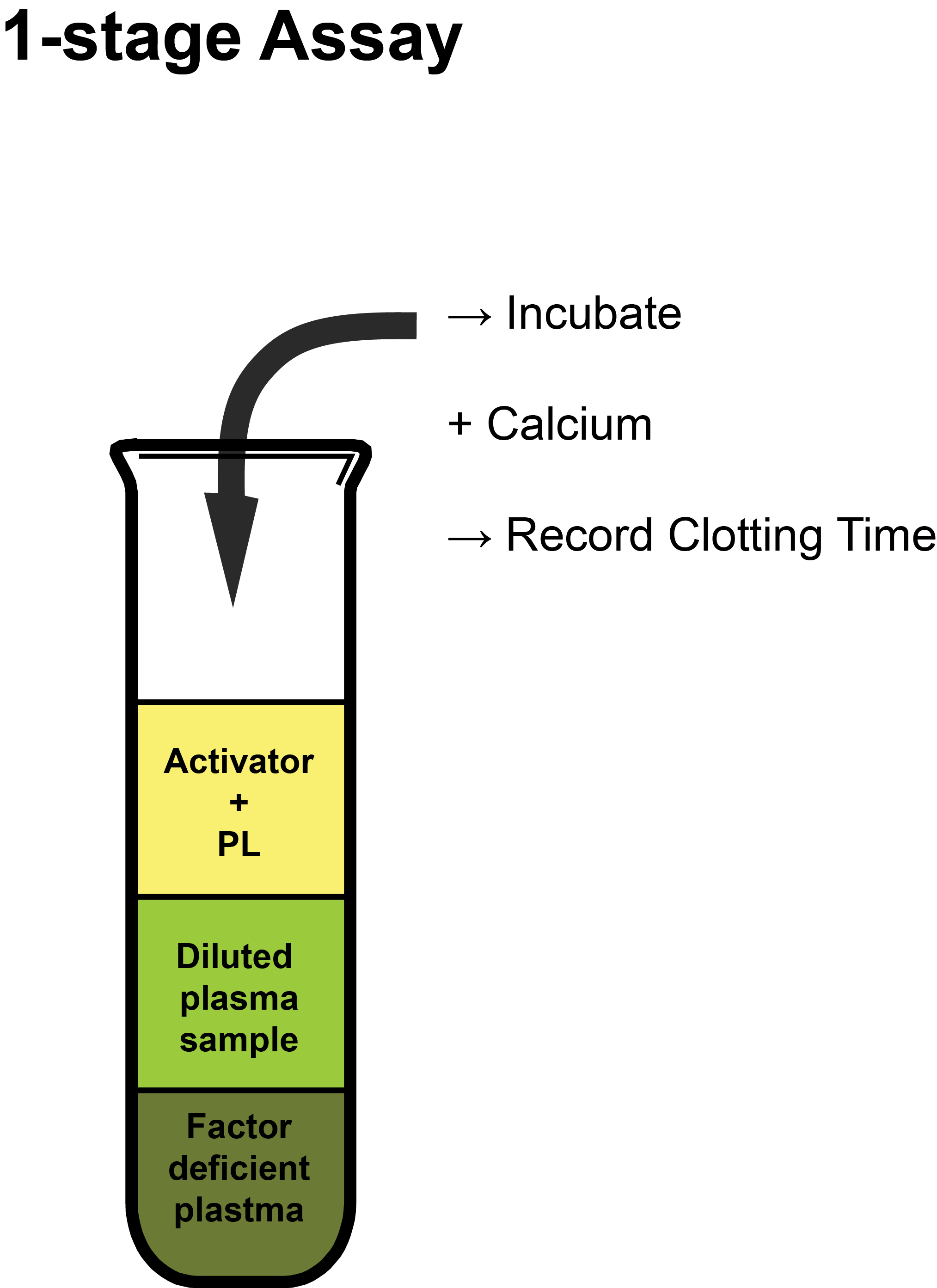
Activator: Depends upon whether the APTT or the PT test is being used for the specific assay. In the case of an APTT-based assay, this is commonly Silica, Ellagic Acid or less frequently Kaolin.
PL: Synthetic Phospholipid used to replace platelet-derived phospholipid as assays are performed on Platelet Poor Plasma [PPP].
Methodology
The reagents used in the assay are summarised below:
| Reagents | |
|---|---|
| APTT Reagents | See APTT |
| Standard Reference Plasma | The reference plasma is used to derive the concentration of the test plasma by comparing the clotting times. Commercial reference standards in which the levels of the clotting clotting factors, are calibrated against international standards and their levels are reported in IU/dl or IU/ml and not %. |
| Factor deficient plasma (substrate plasma) | A plasma deficient in the clotting factor to be assayed - most commonly VIII, IX, or XI. The factor deficient plasma should have a level of the factor being assayed of <0.01 IU/mL and normal levels of all the other clotting factors that affect the APTT test. |
| Patient Plasma | Platelet Poor Plasma [PPP] |
In general [but not always] a 1/10 dilution is taken to represent 100 IU/dL [100% or 1.00 IU/mL]. The following table illustrates the concentrations of a clotting Factor with increasing dilutions if a 1/10 dilution is assumed to have 100% [100 IU/dL or 1.00 IU/mL].
| Dilutions | 1/10 | 1/20 | 1/30 | 1/40 | 1/80 | 1/100 | 1/1000 |
|---|---|---|---|---|---|---|---|
| % Activity | 100% 100 IU/dL 1.00 IU/mL |
50% 50 IU/dL 0.50 IU/mL |
33% 33 IU/dL 0.33 IU/mL |
25% 25 IU/dL 0.25 IU/mL |
12.5% 12.5 IU/dL 0.125 IU/mL |
10% 10 IU/dL 0.10 IU/mL |
1% 1 IU/dL 0.01 IU/mL |
An equal volume [commonly 0.1ml] of the test or reference plasma is incubated with an equal volume of the factor deficient plasma at 37°C. An APTT test is then
performed on each of the mixtures. The clotting times for each dilution are recorded - see below.
In the table below are the data for a Factor VIII assay...
Raw Data
| Sample | Dilutions | |||
|---|---|---|---|---|
| 1/10 [100% FVIII Activity] |
1/20 [50% FVIII Activity] |
1/50 [20% FVIII Activity] |
1/100 [10% FVIII Activity] |
|
| APTT [s] Reference Plasma [FVIII:C = 95 IU/dL] |
28s | 32s | 38s | 42s |
| APTT [s] Patient Plasma | 55s | 59s | 73s | 80s |
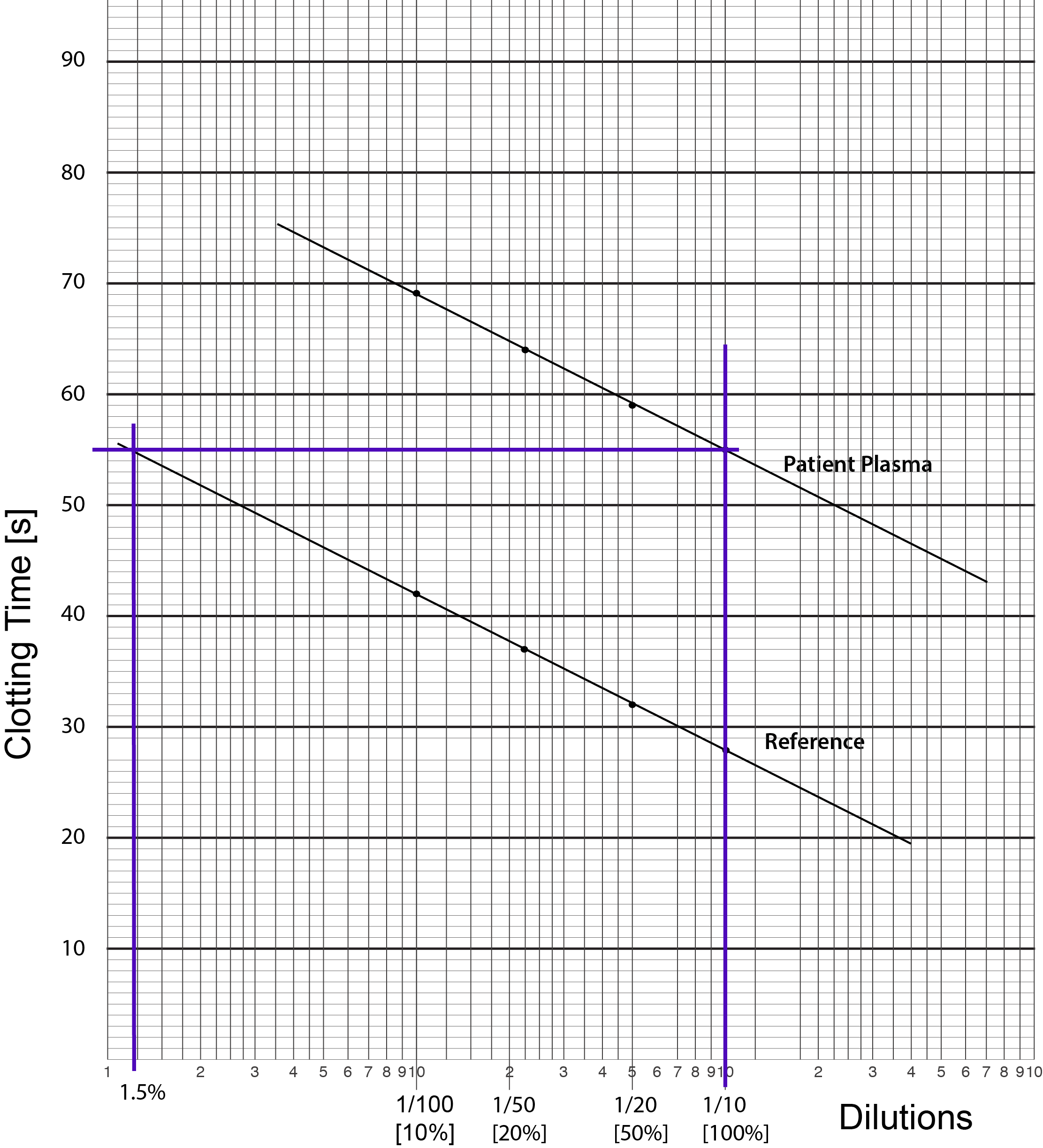
1. Serial dilutions of the Reference (i.e. normal) plasma in buffered saline are mixed with an equal volume of substrate [factor deficient] plasma (i.e. plasma which has normal levels of all the clotting factors other than the clotting factor that is being assayed - in this case Factor VIII - and so the plasma is deficient in Factor VIII ) and an APTT performed. At least three dilutions of both the Reference Plasma and the Test Plasma are required for a factor assay. Additional dilutions may be required if the clotting factor levels fall outwith the range of the assay i.e. the test clotting factor level is very high or very low.
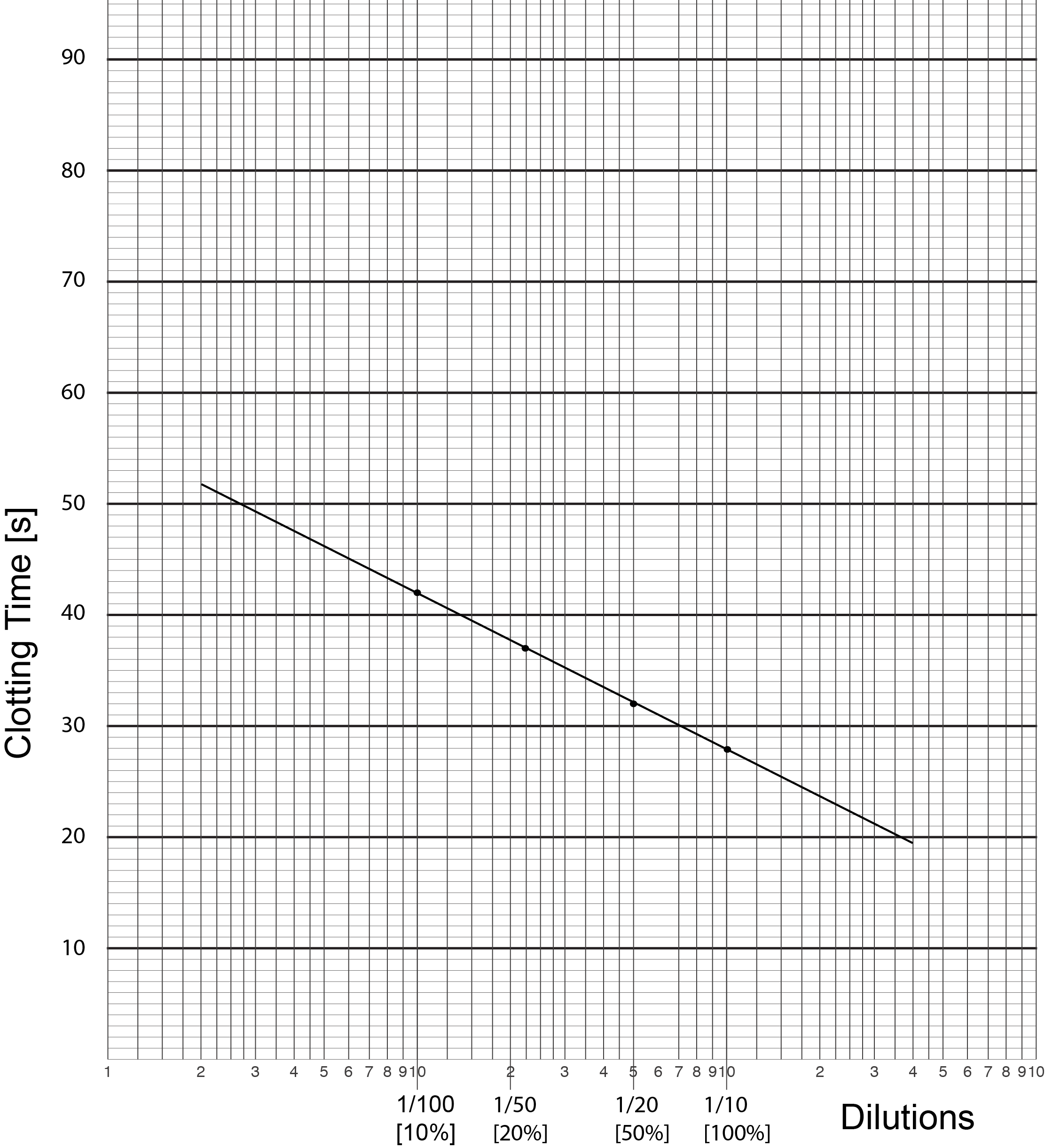
2. The clotting times [in seconds] for the APTTs of the reference plasma are then plotted against dilutions on Log-Lin graph graph and a best fit line drawn through them. The dilutions are plotted on the X axis and the clotting times [s] on the Y axis.
3. This is an example of Log-Lin paper. The log scale is on the X axis [as for PT-based assays] and the Linear scale is on the Y axis.
3. The clotting times [in seconds] for the APTTs of the reference plasma are then plotted against dilutions on Log-Lin graph graph and a best fit line drawn through them. The dilutions are plotted on the X axis and the clotting times [s] on the Y axis.
4. The test plasma is treated the same way as the reference plasma with serial dilutions mixed with equal volumes of substrate plasma and an APTT performed. Since the substrate plasma contains no Factor VIII but provides normal amounts of the other factors the only significant difference between the test plasma dilutions and the standard reference plasma dilutions is the Factor VIII level of the test sample. The clotting time results are plotted on Log-Lin Graph paper
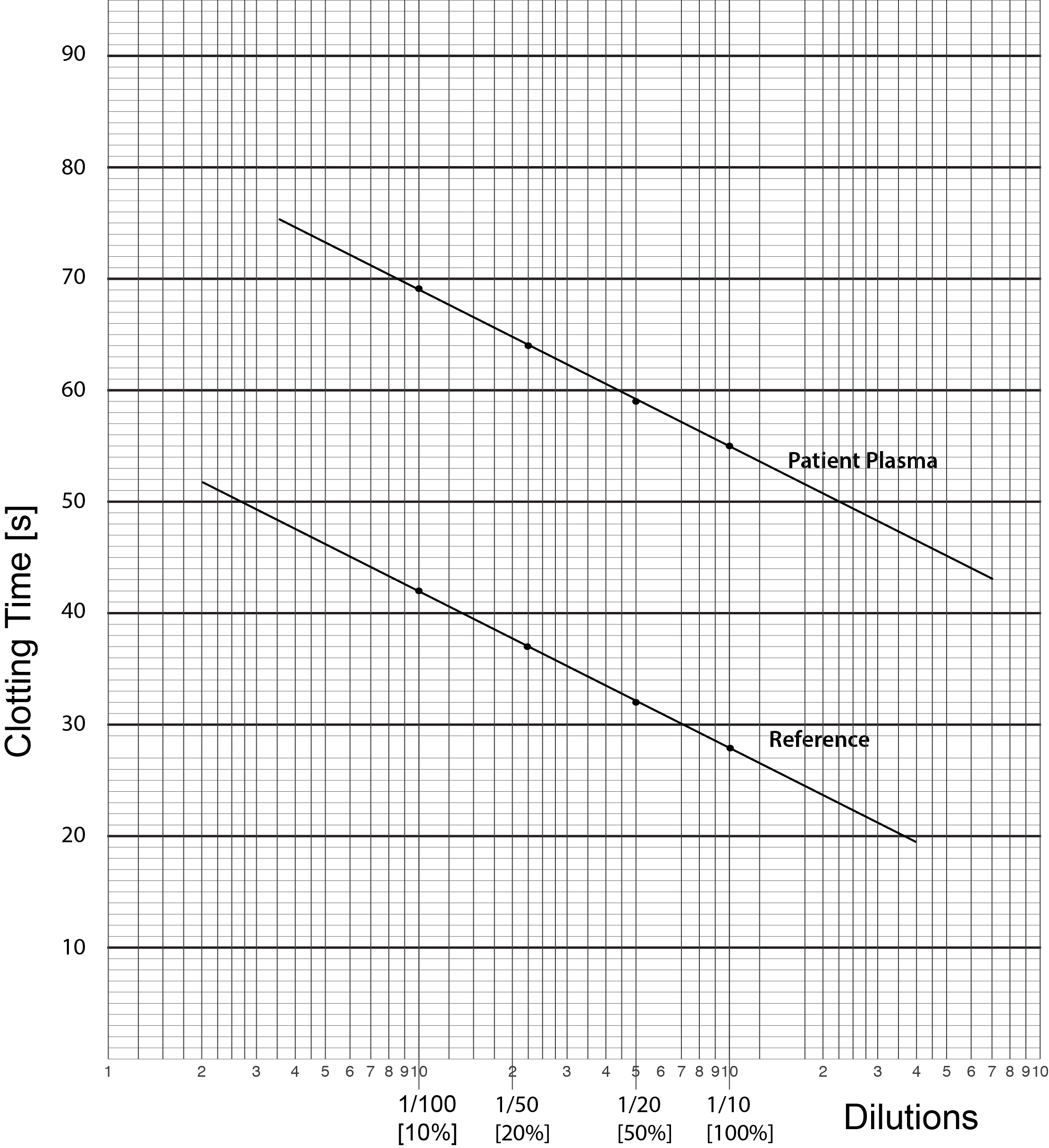
The result should be a straight line running parallel to the line for the standard reference sample unless the Factor VIII level in our test sample is exactly the same as that in the standard sample in which case the lines will be superimposed. If the lines are not parallel then there are several possibilities:
i) The data has not been plotted correctly.
ii) An inhibitor, e.g. a lupus anticoagulant or a Factor inhibitor] is present. In cases in which an inhibitor is present, dilution of the test plasma sample may dilute the inhibitory antibody such that its effect is minimised and the underlying clotting Factor levels can be assayed. Similarly in the presence of a Lupus Anticoagulant the use of Lupus Anticoagulant insensitive reagents may allow the accurate measurement of the clotting factor.
However, you should remember that you cannot dilute nothing and so if the factor level is <1 IU/dL [< 0.01 IU/mL or <1%] you may have non-parallel lines. In this case the clotting times are usually significantly prolonged and identical irrespective of the dilution.
5. We now derive the Factor VIII clotting activity in the Test Plasma sample. To do this we draw a vertical line from the 1/10 dilution through both the Reference and the Test lines. We then draw a line at right angles to this vertical line [i.e. parallel to the X-axis] from when the vertical line intersects the Test plasma until it intercepts the Reference plasma - see below. Where it intercepts the Reference Plasma we then draw another vertical line until it intercepts the X-axis. We then read off the factor level [Factor VIII in this case] from the X-axis. In this case, the line intercepts the X-axis at approximately 1.5% BUT remember the concentration of FVIII in the Reference Plasma is 95 IU/dL. We therefore have to make a correction for this: [95 x 1.5]/100 = 1.4 IU/dL. The final concentration of Factor VIII in the Test Plasma sample is, therefore,1.4 IU/dL.
Interpretation
In general, but not always, the level of Factors VIII and IX correlate with bleeding risk. However it should be remembered that some F8 gene mutations are associated with discrepant Factor VIII levels depending upon how the Factor VIII is measured - see 2-stage FVIII assays and Chromogenic FVIII assays.
| Severity | Factor Levels |
|---|---|
| Severe Haemophilia A or B | Factor VIII or IX <0.01 IU/mL [<1 IU/dL [%]] |
| Moderate Haemophilia A or B | Factor VIII or IX is >0.01 IU/mL [>1 IU/dL [%]] but <0.05 IU/mL [<5 IU/dL [%]] |
| Mild Haemophilia A or B | Factor VIII or IX is 0.05-0.30 IU/mL [5-30 IU/dL [%]] |
In Factor XI deficiency the bleeding risk varies between individuals with the same Factor level but is consistent for a given individual. There is data that analysing Thrombin Generation in patients with Factor XI deficiency can be of value in establishing their bleeding phenotype - see References.
Failure to obtain a straight line or non-parallel lines when plotting the APTT/dilution of the test sample may indicate the presence of an inhibitor.
Plot your own data...
The data below will allow you to plot a number of factor VIII assays. The clotting time for each dilution is shown in seconds [s]. Click HERE for a pdf of Log-Lin graph paper.
| Plasma Source | Dilution |
|||||
|---|---|---|---|---|---|---|
| 1/5 | 1/10 | 1/20 | 1/40 | 1/80 | 1/100 | |
| APTT [s] Standard Reference Plasma [FVIII:C Reference Plasma 94 IU/dL] |
41s | 53s | 66s | 82s | ||
| Plasma Sample 1 | 55s | 79s | 96s | |||
| Plasma Sample 2 | 108s | 120s | ||||
| Plasma Sample 3 | 70s | 82s | 107s | |||
| Plasma Sample 4 | 30s | 42s | 55s | 71s | ||
The graph shows the results of the Factor VIII assays. The reference plasma standard is plotted in red. Note that the lines are parallel.
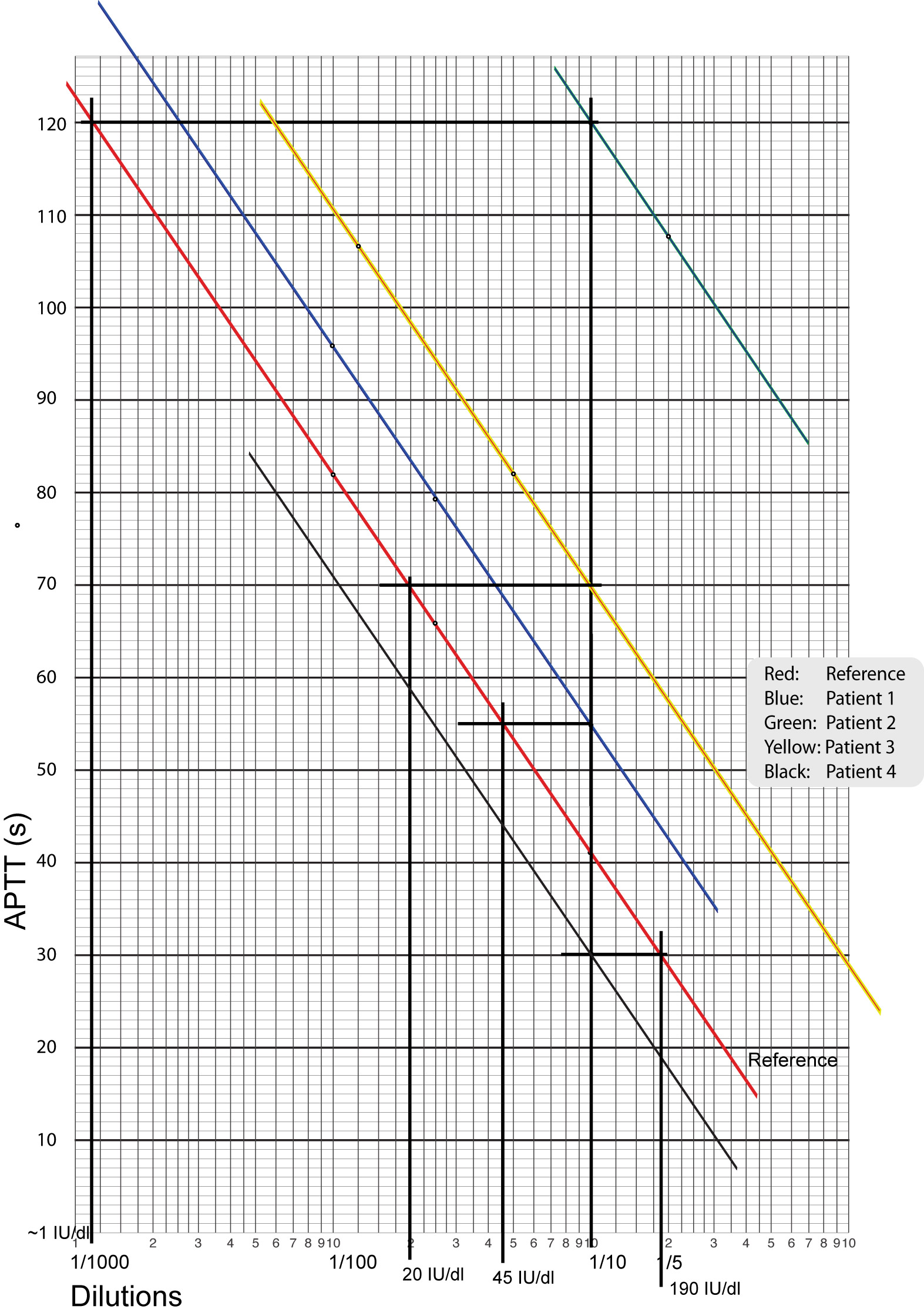
| Plasma Sample | Factor VIII Levels [IU/dL] [Reference plasma FVIII concentration 94 IU/dL] |
Factor VIII Levels [IU/mL] [Reference plasma FVIII concentration 0.89 IU/mL] |
|---|---|---|
| Plasma Sample 1 | 43 IU/dL | 0.40 IU/mL |
| Plasma Sample 2 | 1 IU/dL | 0.01 IU/mL |
| Plasma Sample 3 | 19 IU/dL | 0.18 IU/mL |
| Plasma Sample 4 | 179 IU/dL | 1.70 IU/mL |
Don't forget that you need to make a correction for the Factor VIII concentration in the Reference plasma 94 IU/dL.
If the FVIII reference plasma concentration is 0.89 IU/mL - what are that results for the 4 plasma samples?
Reference Ranges
Reference ranges should be expressed as IU/mL but in practice many are still reported as as either IU/dL or %. Many factors, including Factors VIII, IX, X, have reference ranges of 0.50-1.50 IU/mL [50-150 IU/dL or 50-150% but in practice the reference ranges vary significantly. The range for Factor XI is narrower at 0.65 – 1.25 IU/L.
Reference ranges for a given factor do not, in general, change with age above 6 months. Neonates have physiologically lower normal ranges due to immaturity of the fetal/neonatal liver - see section on Paediatric Reference Ranges.
In addition, Factor VIII is an acute phase protein and so its levels will increase in response to stress including pregnancy and surgery. This can lead to shortening of the APTT and in some cases may mask an otherwise prolonged APTT due to another clotting factor deficiency e.g. Factor XI.
What Test Next?
The following table provides a summary of what tests might be undertaken finding an abnormal Factor assay.
| Specific Factor Deficiency | |
|---|---|
| Factor VIII | 1. If Factor VIII deficiency is a new and unexpected finding then Von Willebrand Factor levels and Factor V levels should be checked. This is unnecessary if a finding of Factor VIII deficiency is expected (e.g. a known individual with Haemophilia A). 2. There are an increasing number of cases in whom patients with Factor VIII deficiency have discrepant Factor VIII levels when measured by a 1-stage assay compared to a 2-stage or chromogenic FVIII assay. The bleeding phenotype correlates better with the 2-stage or chromogenic FVIII assay. It is essential, therefore, that all individuals with a suspected bleeding disorder have both a 1-stage and chromogenic Factor VIII assay performed to identify those individuals with a normal 1-stage Factor VIII assay [and therefore a normal APTT] but a reduced Factor VIII level measured by the chromogenic assay. If both tests are not performed then some individuals may be misdiagnosed either as 'normal' or with a reduced factor VIII level but the assay result does not correlate with their bleeding phenotype. 3. Acquired Factor VIII deficiency. An otherwise healthy individual may develop an auto-antibody against Factor VIII leading to acquired haemophilia A. Such antibodies are also seen in patients with a number of immunological disorders e.g. Rheumatoid arthritis. 4. A low FVIII can also be found in von Willebrands Disease [VWD] and in Acquired Von Willebrand Syndrome [AVWS]. 5. Factor VIII is an acute phase protein and the levels may be high in individuals who are stressed and this includes pregnancy. This may lead to shortening of the APTT. 6. The concentration of FVIII is a major determinant of the APTT and low FVIII levels will prolong the APTT and conversely high FVIII levels will shorten the APTT. 7. In a female with a low FVIII or FIX and no relevant family history, the karyotype should be established e.g. Turners syndrome should be considered. |
| Factor IX | Low Factor IX levels are seen in Haemophilia B. Acquired FIX inhibitors [autoantibodies] are rare. Factor IX is a vitamin K dependent clotting factor and therefore will be reduced in association with other factor deficiencies [II, VII and X] in cases of Vitamin K Deficiency. There are other Vitamin K dependent proteins e.g. Protein C, S and Z that will also be reduced in cases of Vitamin K deficiency. |
| Factor X | If Factor X deficiency is suspected but an APTT- based assay does not give the expected results consider using an alternative technique (e.g. PT based assay, a chromogenic assay, immunoassay) as mutations causing deficiencies exist which are detectable only with certain techniques. Acquired FX inhibitors are – rare but a low Factor X level may be seen in some patients with amyloidosis. |
| Factor XI | FXI deficiency is rare but significantly more common in individuals of Jewish origin. In individuals of Jewish origin, four mutations within the Factor XI [FII] gene [Types I - IV] account for the majority of cases of Factor XI deficiency: Type I: GTA-ATA Exon 14/Intron boundary Type II: GAA-TAA Exon 5 Type III: ATT-ATC Exon 9 Type 4: 14bp deletion Exon 14/intron boundary |
| Any factor deficiency | Consider: 1. Family screening and ensure you have constructed a family pedigree 2. Mutation analysis |
