Thrombin Activatable Fibrinolysis Inhibitor [TAFI] Assays
Introduction
TAFI is an enzyme that circulates in plasma and is involved in the regulation of fibrinolysis. TAFI when activated to TAFIa, functions to suppress fibrinolysis by the removal of C-terminal lysines from the fibrin clot that are exposed as fibrin [in the fibrin clot] is degraded by Plasmin. In this way tissue Plasminogen Activator[t-PA] binding is restricted and further activation of Plasminogen to Plasmin, prevented.
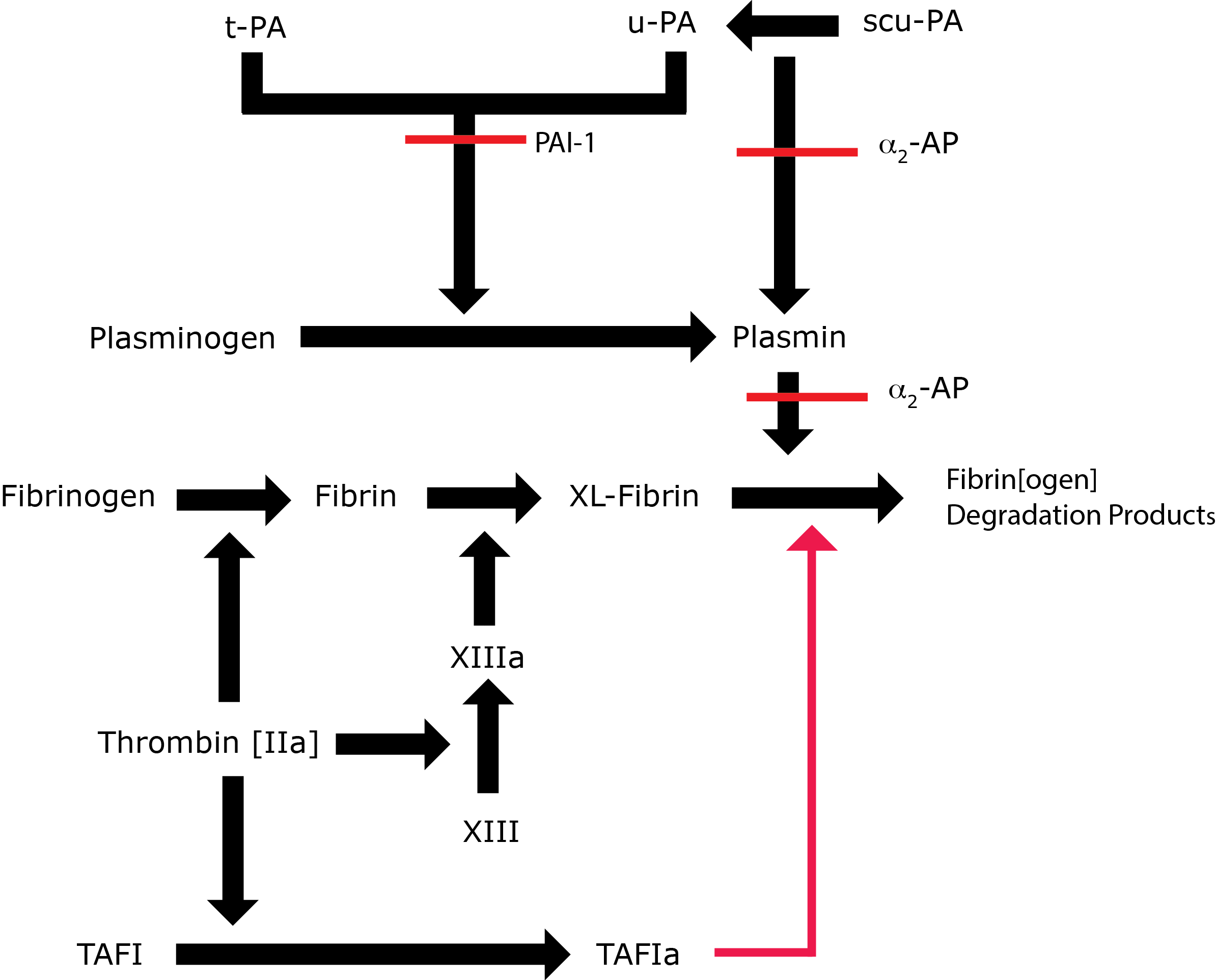
Key: t-PA - Tissue Plasminogen Activator; u-PA - Urokinase-type Plasminogen Activator; XL-Fibrin - cross-linked fibrin; TAFI - Thrombin Activatable Fibrinolytic Inhibitor; TAFIa - Activated Thrombin Activatable Fibrinolytic Inhibitor; α2-AP - Alpha-2-antiplasmin [Alpha2-Plasmin Inhibitor]; scu-PA - single chain Urokinase-type Plasminogen Activator. A red line indicates an inhibitory pathway.
TAFI is synthesised in the liver [and possibly in platelets] as a propeptide of 423 amino acids comprising a 22 amino acid signal peptide, a 92 amino acids activation peptide and a 309 amino acids catalytic domain..
Activation of TAFI to generate the active form occurs by cleavage of the protein at Arg92 with the release of a 19kDa activation peptide. Activated TAFI [TAFIa] has a T½ of several hours.
Fibrinolysis is initiated after the formation of a fibrin clot. Ordinarily the inactivation of Plasminogen to Plasmin by t-PA occurs relatively slowly and inefficiently but in the presence of fibrin both Plasminogen and t-PA bind to the fibrin leading to the formation of a ternary complex and a significant increase in the rate of conversion of Plasminogen to Plasmin. In addition, Plasmin bound to the fibrin clot is protected from inactivation by α2-Plasmin Inhibitor [α2-Antiplasmin]. As the bound Plasmin cleaves fibrin it generates new C-terminal lysine residues that further enhance plasminogen binding and activation to Plasmin .
TAFIa inhibits fibrinolysis by removing the C-terminal lysine residues from fibrin and so prevents the binding of Plasminogen and t-PA to the fibrin clot. In addition TAFIa also inhibits the activation of Glu-Plg to Glu-Plasmin and the conversion of Glu-Plg to Lys-Plg.
Activation of TAFI to TAFIa by thrombin is relatively inefficient in the absence of Thrombomodulin [Tm] – Tm increases the rate of activation ~1000-fold.
Relatively high thrombin concentrations are required to convert TAFI to TAFIa in contrast to the relatively low concentrations of Thrombin required to cover Fibrinogen to Fibrin. For these reasons activation of TAFI occurs through the so-called intrinsic pathway of coagulation via Factor XI [FXI]. A deficiency or functional abnormality of TAFI may be important in contributing towards the bleeding phenotype seen in patients with FXI deficiency and may also contribute to the severity of the bleeding in FVIII and FIX deficiency. Interestingly the TAFI knock-out mouse model shows normal embryonic development, normal fertility and no apparent increase in haemorrhagic or thrombotic disorders. However, at least in some K/O mouse models they show defective wound healing suggesting that TAFI is involved in tissue repair.
A single nucleotide polymorphism [SNP] in the TAFI gene results in the substitution of a Threonine by an Isoleucine at position 325 and this substitution doubles the T½ of TAFI [from 8 to 15 minutes at 37°C] and results in increased fibrinolytic activity. However, this SNP also appears to be associated with reduced TAFI immunological levels possibly by linkage to other SNPs within the TAFI gene that affect expression of the protein.
Principles & Methods
1. Immunological Assay: A standard ELISA assay can be used to measure TAFI antigen. A microtitre plate is coated with a specific monoclonal antibody to the TAFI antigen and diluted plasma samples or controls are added to the wells. The plate is incubated to allow the antibody to bind to the TAFI and then washed. Bound TAFI is demonstrated by a adding a second anti0-human TAFI antibody coupled to horseradish peroxidase (HRP]. Following a further washing step a substrate [tetramethylbenzidine – TMB] is added to the wells which in the presence of hydrogen peroxide generates a blue colour change. The addition of sulphuric acid stop the reaction and turn the blue to yellow. The absorbance at 450nm is measured and the absorbance is directly proportional to the TAFI concentration in the sample.
2. Functional Assays:
A.
Retardation of clot lysis by TAFIa – this assay is based on the ability of TAFIa to delay the lysis of a plasma clot induced by recombinant t-PA [rt-PA]. rt-PA is used to induce the formation of a fibrin clot. TAFI is activated by Thrombin and Tm and the [functional activity of] TAFI present in the plasma sample leads to an inhibition of fibrinolysis which can be measured by the change in OD. In the presence of low or functionally abnormal TAFI, then impairment of fibrinolysis does not occur and this can be detected by a more rapid change in OD.
B. Plasma is activated with Thrombin-Thrombomodulin. After 10 minutes the reaction is stopped by the addition of PPACK [a protease inhibitor] and the TAFIa is measured by a specific substrate [Hippuryl-Arg].
Interpretation
1. Bleeding: A deficiency or functional abnormality of TAFI may be important in contributing towards the bleeding phenotype seen in patients with FXI deficiency and may also contribute to the severity of the bleeding in FVIII and FIX deficiency.
2. Elevated TAFI levels may contribute to an increased risk of venous thrombosis compared to TAFI levels within the reference range.
Reference Range
TAFI:Ag varies widely in the healthy population. There does not appear to be a gender difference and levels do not appear to change in pregnancy. The concentration of TAFI is ~2-fold higher in fetal than maternal blood.
Normal TAFI:Ag results are in the region of 5.8-10.0µg/ml [100-172nM.]
What Test Next
In individuals with an abnormal TAFI or disordered fibrinolysis, it may be useful to undertake specific assays of the components of the Fibrinolytic pathway, to perform a TEG/ROTEM or to sequence the relevant genes.
