α2-Plasmin Inhibitor Assays
[Also known as α2-Antiplasmin]
Introduction
α2-Plasmin Inhibitor [previously known as α2-Antiplasmin [ α2-AP] in conjunction with TAFI and PAI-1 is the primary physiological inhibitor of Plasmin and therefore plays a critical role in the regulation of fibrinolysis. α2-PI is synthesised by the liver but is also found in the α-granules of platelets and so secreted when platelets are activated and more recently has also been found in the kidney and brain.
α2-Plasmin Inhibitor is a member of the SERPIN family of proteins.
Two forms of α2-PI circulate in plasma:
1.
A 464-residue protein with methionine at the N-terminus [Met-α2-PI]
2.
An N-terminally shortened form [452 amino acids] in which the N-terminal amino acid is an asparagine residue [Asn-α2-PI].
Approximately 30% of the α2-Plasmin Inhibitor in the plasma is found as Met-α2-PI and 70% as Asn-α2-PI. Asn-α2-PI appears to be more physiologically active than Met-α2-PI.
Fibrinolysis is regulated by α2-Plasmin Inhibitor [α2-PI] in 3 ways:
1. By the formation of a stoichiometric complex with Plasmin. α2-PI is much more efficient at inhibiting free Plasmin than Plasmin bound to the fibrin clot and this allows localised Plasmin generation on the fibrin clot while preventing/inhibiting systemic enzyme activation.
2. By the inhibition of Plasmin adsorption on the fibrin clot. α2-PI is covalently bound into the fibrin clot by FXIIIa, resulting in increased resistance to fibrinolysis by the fibrin clot.
3. By preventing the binding of Plasminogen into the fibrin clot. The C-terminus of α2-Plasmin Inhibitor binds with strong affinity to the lysine binding sites of Plasminogen where fibrin also binds. In this way α2-PI prevents Plasminogen binding to fibrin.
α2-PI accounts for approximately 90% of Plasmin inhibition in vivo. Plasmin is also inactivated by α2-macroglobulin.
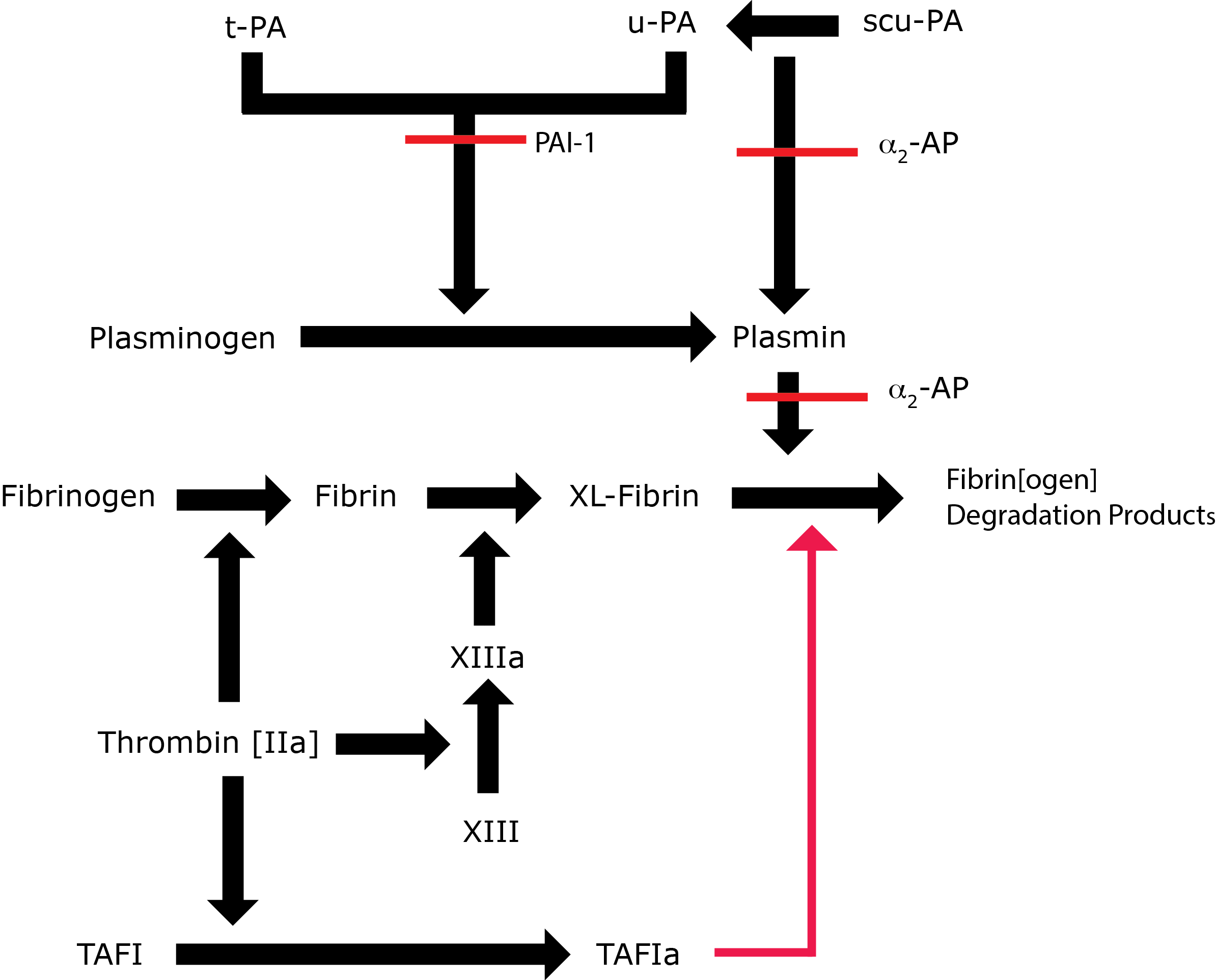
Key: t-PA - Tissue Plasminogen Activator; u-PA - Urokinase-type Plasminogen Activator; XL-Fibrin - cross-linked fibrin; TAFI - Thrombin Activatable Fibrinolytic Inhibitor; TAFIa - Activated Thrombin Activatable Fibrinolytic Inhibitor; α2-AP - Alpha-2-antiplasmin [Alpha2-Plasmin Inhibitor]; scu-PA - single chain Urokinase-type Plasminogen Activator. A red line indicates an inhibitory pathway.
Principles & Methodology
α2-Plasmin Inhibitor can be assayed either immunologically or functionally:
1. Immunological α2-PI assays.
These are commonly performed using an ELISA assay in which an antibody to α2-PI is immobilised onto a microtitre plate and then used to capture the α2-PI.
A recently reported method for measuring α2-PI utilises an ELISA assay for the determination of free total α2-PI in plasma. A biotinylated monoclonal antibody recognizes and captures all the four N-and/or C-terminally modified isoforms of α2-PI and a Horse Radish Peroxidase [HRPO]-labelled polyclonal anti-α2-PI antibody is then used to the captured antigen.
A latex-based method for measuring α2-PI has also been reported.
2. Functional assays:
a.
Patient plasma containing α2-PI is incubated with Plasmin in excess. The Plasmin is rapidly inactivated by the α2-PI and residual Plasmin is measured in an amidolytic assay in which the residual Plasmin cleaves a chromogenic substrate and the change in colour is detected by measuring the changes in absorption at 405nm. The change in absorption is inversely proportional to the concentration of α2-PI.
b. Plasmin is coated onto a microtitre plate and plasma samples then added to the plate. Functionally active α2-Plasmin Inhibitor binds to and reacts with the Plasmin. The wells are washed and an anti-α2-Plasmin Inhibitor added to the wells. The bound antibody is detected using horseradish peroxidase conjugated to a second antibody. The wells are washed and then TMB [a substrate for the horseradish peroxidase] added and the colour change at 405nm is recorded. The amount of colour change is proportional to the concentration of active α2-PI in the original plasma sample.
Interpretation
Inherited α2-Plasmin Inhibitor deficiency is rare and only a small series of case reports exist in the literature. Its importance lies in the fact that the normal screening tests of coagulation e.g. PT and APTT are normal in cases of α2-Plasmin Inhibitor deficiency but the TEG/ROTEM may be abnormal.
Patients with homozygous deficiencies of α2-PI usually have values <0.10 IU/mL of normal and such cases present with bleeding. Heterozygous individuals with levels of α2-PI in the region of 0.30 - 0.60 IU/mL are usually asymptomatic.
Reference Ranges
The normal adult reference range is 0.80 - 1.20 IU/mL.
Healthy full-term infants may have borderline-low or mildly decreased levels of α2-PI but these generally reach adult levels by the end of the first week of life. Premature infants may have low levels that do not reach adult values for up to 90 days.
What Test Next
The TEG/ROTEM may be abnormal in cases of suspected α2-PI and may be a useful screening test for this disorder. Consider sequence analysis of the relevant genes if disordered fibrinolysis is suspected.
