Introduction
Protein S is a vitamin K-dependent glycoprotein of 635 amino acids, MW of 70KDa and is a key component of the Protein C-Protein S natural anticoagulant pathway.
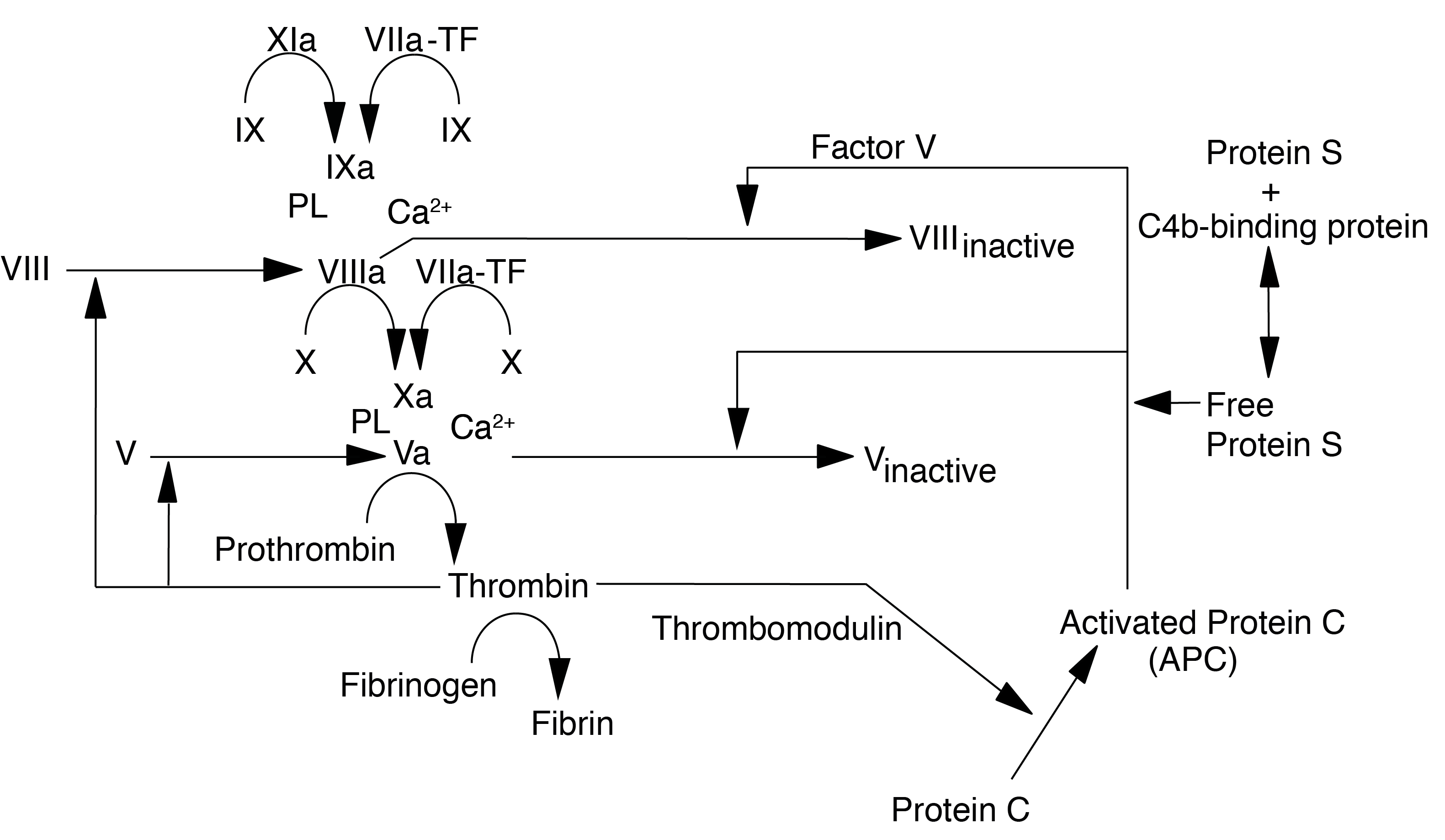
Protein S acts as a cofactor for the serine protease Activated Protein C [APC] in the inactivation of Factors Va and VIIIa. Protein S also has APC-independent anticoagulant activity through direct binding to Factor Va, Factor Xa and Factor VIII. In addition to its anticoagulant role, Protein S plays a role in enhancing the phagocytosis of apoptotic cells.
Protein S exists in 2 forms – bound and free. About 65% is in the bound form, complexed to C4b-binding Protein (C4bBP) and is functionally inactive. Only the free form has activity. The proportion of bound and unbound forms is regulated by the availability of C4bBP. Protein S is synthesised in the liver, endothelial cells and megakaryocytes and has a T½ of 42 hours.
Protein S deficiency is inherited in an autosomal dominant manner and increases the risk of VTE. Homozygous Protein S deficiency may present in newborns with purpura fulminans (a form of disseminated intravascular coagulation characterised by extensive cutaneous haemorrhage and necrosis) which is rapidly fatal unless treated with Protein S replacement [usually Fresh Frozen plasma as no Protein S concentrate currently exists although Prothrombin Complex Concentrates [PCCs] contain in addition to Factors II, VII, IX and X - Proteins C and S.
There are three types of hereditary Protein S deficiency:
Type |
Free Protein S |
Bound Protein S |
Total Protein S |
Protein S Function |
|---|---|---|---|---|
I |
↓ |
↓ |
↓ |
Normal |
II |
Normal |
Normal |
Normal |
↓ |
III |
↓ |
↑ |
Normal |
Normal |
However, it is probable that Type I and III are phenotypic variants of the same genetic mutation and the sub-division into Types I and III at least in some individuals, may be inappropriate. Protein S levels increase with age and this may in part explain this phenotypic variation.
Many cases of Type II deficiency have on re-investigation been found to be due to the presence of the Factor V Leiden mutation.
Principles
Assays for Protein S [PS] can be either functional or immunological.:
1. Functional assays measure only Free [physiologically-active] Protein S. However, Protein S activity assays are sensitive to the presence of Lupus Anticoagulants and both acquired and inherited APC resistance.
2. Total Protein S Antigen [PS:Ag]: Techniques for measuring Protein S antigen include ELISA and radio-immunoassays. ELISA is the most commonly used and a number of commercial kits are available. Whatever method is used it must assay Protein S independently of the levels of C4bBP.
3. Free Protein S Antigen: This requires that the C4bBP-bound Protein S is removed from the assay. This can be achieved either by:
a.
A pre-assay step that involves precipitating the C4bBP-bound Protein S with polyethylene glycol [PEG] then removing the precipitate by centrifugation. The remaining Protein S in the plasma sample is free, unbound Protein S.
b.
An alternative is to use a monoclonal antibody directed against the Protein S epitopes that are not accessible in the bound form and these are usually the residues that are involved in the binding to C4bBP.
Immunological assays will measure both free and bound PS i.e. Total PS. There is a good correlation between Free Protein S antigen and functional PS activity and so many labs choose only to measure Free Protein S antigen. However this may miss some rare cases in which the immunological PS level is normal but there is a functional abnormality i.e. a true Type 2 deficiency. In these rare cases, mutational analysis of the genes involved in haemostasis will identify these cases and may be a more logical approach rather than establishing a functional PS assay.
Method
1. Immunological Assays:
| Assay | Description |
|---|---|
| a. ELISA Assays | Commonly ELISA-based assays are used in which the wells of a microtitre plate are coated with rabbit or goat anti-human PS antibodies. Wells are incubated with plasma and after washing a second diluted anti-PS conjugated to horseradish peroxidase [HRP] is added. The amount of HRP bound antibody is measured with a third incubation with a specific substrate for HRP. The degrees of absorbance is measured. A calibration is curve is constructed using a know concentration of PS and from which the concentration of PS in the unknown plasma sample can be determined. See references for more information on the principles of ELISA assays. |
| b. Latex particle-based Agglutination Assays | In this assay purified C4BP is adsorbed onto the first latex reagent and then incubated with the patient plasma. Free PS is adsorbed on the C4BP latex particle and this triggers an agglutination reaction with the second latex reagent which is sensitised with a monoclonal antibody directed against human Protein S. The degree of agglutination is directly proportional to the free Protein S concentration in the test sample. |
2. Functional Assays: These are usually based on either the PT or the APTT:
| Functional Assay | Description |
|---|---|
| a. APTT-based Functional Protein S Assays | Patient platelet poor plasma is incubated at 37°C with Protein S deficient plasma, phospholipid, a contact activator (e.g. Kaolin) and either an excess of activated Protein C [APC] or an activator of Protein C (e.g. Protac). After incubation (typically 1-4 minutes) calcium is added to initiate clotting. The time taken to form a clot is timed. From this the Protein S level is determined by comparison to a reference curve created using dilutions of a reference plasma of known PS concentration. Activated Protein C together with its co-factor Protein S, will inactivate FVIIIa and FVa. The levels of Protein S will, therefore, influence the clotting times. |
| b. PT-based Functional Protein S Assays | The APC co-factor activity of PS is measured as the degree of prolongation of a PT-based clotting time. PS depleted plasma is mixed with the test plasma sample and Protac-activated PC, Tissue Factor, phospholipid and calcium ions are mixed. The prolongation of the clotting time is proportional to the concentration of Protein S in the plasma sample. The Protein S level is determined by comparison to a reference curve created using dilutions of a reference plasma of known PS concentration. Activated Protein C together with its co-factor Protein S, will inactivate FVa. The levels of Protein S will, therefore, influence the clotting times. |
Interpretation
1. Homozygous Type I Protein S deficiency is easily diagnosed in the neonate as Protein S levels are usually undetectable at presentation. However, the reference range for neonates is very wide and heterozygous or Type II and III deficiency may require repeat testing at 6 months to detect. If this is impractical assays of other vitamin K-dependent coagulation Proteins for comparison or measurement of parental levels of Protein S may be helpful.
Mutational analysis of the Protein S gene - PROS1 - can also be of value in these rare cases.
2.
Type II deficiency is very rare but a functional assay for Protein S deficiency may be helpful if there is a high index of suspicion, other tests are normal, interfering factors can be excluded and the assay can be reliably performed.
Again PROS1 mutational analysis can be of value in these rare cases.
3.
Functional PS assays may result in
misleadingly low Protein S levels in the presence of Factor V Leiden and some other causes of Activated Protein C resistance including elevated Factor VIII levels.
4.
Direct thrombin inhibitors and other anticoagulants acting through pathway inhibition also interfere with the Protein S functional assay.
5. Protein S levels are low in pregnancy and in some women on the OCP.
6. Protein S levels are reduced in patients with vitamin K deficiency and or taking a vitamin K antagonist e.g. Warfarin.
7. Protein S levels may fall in patients with liver disease but this is not universal as there are extra-hepatic sites of Protein S synthesis.
8. Protein S deficiency has been reported in association with HIV infection.
9. Life-threatening purpura fulminans due to acquired Protein S deficiency has been reported in association with chickenpox (varicella).
10. Protein S levels may fall in Sickle Cell disease and this has been reported to be due to absorption of PS by the sickle red cells. Interestingly Protein S levels do not fall excessively during a sickle crisis.
11. Warfarin-induced skin necrosis has been reported in patient with Protein S deficiency although the mechanism is unclear as the half-life of Protein S is long compared to the other procoagulant factors or Protein C.
Reference Ranges
The reference range for Protein S are:
Males >70-140 U/dL.
Females >60-130 U/dl.
Protein S levels are low at birth [as are all the Vitamin K dependent clotting factors] and do not reach adult values until approximately 6 months of age. See Paediatric Haemostasis.
What Test Next
In cases of Protein S deficiency, acquired causes should be excluded. In cases of apparent neonatal purpura fulminans, the parents should be screened.
Mutational analysis of the Protein S gene - PROS1 - should be undertaken in all cases of Protein S deficiency. This can be invaluable in genetic counselling based upon the natural history of similar mutations.
