2. Rotational Thromboelastometry - ROTEM®
Introduction
Thromboelastography was first described by Hartert in 1948 - see References. Thromboelastography (TEG®) and Thromboelastometry (ROTEM®) provide global information on the dynamics of clot development, stabilisation and dissolution that reflect in vivo haemostasis. Thromboelastography is increasingly used as a Point of Care Test [POCT] in a number of situations and these include:
1. Trauma:
Thromboelastography is commonly used in the trauma setting - see References for further information.
2. Major surgery [including hepatic surgery and transplantation], major blood loss and transfusion.
Thromboelastography is used as a POCT monitor in complex surgery and has been shown to significantly reduce the use of blood component therapy and overall blood loss.
3. Pregnancy:
The
TEG® and ROTEM® are increasingly used in pregnancy and the puerperium. Pregnancy is a hypercoagulable state and this is reflected in the TEG® and ROTEM® parameters. It may not, therefore, be appropriate to use reference ranges for the TEG® and ROTEM® that have been derived from non-pregnant individuals. For further information and for reference ranges that have been derived in pregnancy - see References
4. Assessment of platelet function
5. Hypercoagulable disorders
6. Disseminated Intravascular Coagulation [DIC]
7. The investigation of individuals with an unclassified bleeding disorder
For additional information on viscoelastic elastic assays and emerging technologies - see Hartman et al 2020.
Principles and Methodology
Whole blood is added to a heated cuvette at 37°C. Within the cup a pin is suspended connected to a detector system - this is a torsion wire in the case of the TEG® device and an optical detector in the case of the ROTEM® device. In the TEG® machine the cup oscillates in a limited arc of 4°45' every 5 seconds whereas in the ROTEM® the pin oscillates in a limited arc of 4°75' every 6 seconds. As the blood clots, Fibrin-Platelet strands form between the cup and the pin and as the viscoelastic strength of the clot increases, the rotation of the cup is transmitted to the pin in the case of the TEG® device but in the ROTEM® device it impedes rotation of the pin. This is detected and a trace generated. With the onset of Fibrinolysis, the Fibrin-Platelet structure begins to dissolve affecting movement of the pin and this is again reflected in the trace.
Although the TEG® originally involved fresh whole, non-anticoagulated blood, both the TEG® and the ROTEM® commonly employ citrated whole blood that is re-calcified to initiate coagulation. It is also common to use an activator as this standardises the test and in addition speeds up the rate at which clotting takes place and hence the rate at which a result is generated. The TEG® and ROTEM® devices have a number of separate channels allowing a number of samples to be run simultaneously or sequentially.
1a. Thromboelastogram [TEG®]
The figure below shows the traces/waveforms obtained using the TEG® system. The table summarises the descriptive data reported with both the TEG® and ROTEM® devices.
Variables measured by the TEG® and ROTEM® Devices
| Variable | TEG® | ROTEM® |
|---|---|---|
| Time [minutes] from the start of the test to when the waveform reaches 2mm above baseline. This represents the initiation of coagulation |
Reaction Rate [R] | Clotting Time [CT] |
| Time [minutes] from 2mm above baseline to 20mm above baseline. This represents the amplification process of coagulation, the interaction with platelets and stabilisation of the Fibrin clot by Factor XIII |
Kinetics Time [K] | Clot Formation Time [CFT] |
Alpha [α] angle [°]. |
The angle between the end of the R time to the slope of the developing curve | The angle between the end of the CT to the slope of the developing curve |
| A10 | - | Waveform amplitude 10 minutes after CT |
| Maximum strength [mm] and which measures the maximum displacement of the waveform i.e. the peak amplitude of the clot. This represents the overall strength of the clot and is dependent upon the formation of Fibrin, the interaction with platelets, stabilisation of the Fibrin clot by Factor XIII and the Haematocrit. |
Maximal Amplitude [MA] | Maximal Clot Firmness [MCF] |
| Time [minutes] to Maximum Clot Firmness | - | MCF-t |
| Amplitude [mm] at a specific time point after the start of the end of the R/CT times e.g. 5 minutes [A5], 10 minutes [A10], 30 minutes [A30], 60 minutes [A60] etc | A30, A60 | A5, A10... |
| Clot elasticity [dyn/cm2] - measures the 'firmness' of the clot | G | MCE |
| Maximum Lysis - a measure of the percent of the decrease in amplitude of the waveform at the end of the test | - | ML |
| Clot Lysis at a specific time [minutes] - a measure of the percent of decrease in waveform amplitude at 30 and 60 minutes after the waveform has reached maximum amplitude [MA/MCF]. This measures clot stability and Fibrinolysis. |
CL30, CL60 | LY30, LY45, LY60 |
1b. TEG6s Analyser
The TEG6s analyser uses resonance-frequency viscoelastic measurement and a disposable all-in-one cartridge. The blood sample is suspended in a multi-channel cartridge and then exposed to external vibration (20–500 Hz). As clotting proceeds, the changes in the clot-strength-specific resonance frequencies are monitored and converted into TEG-equivalent units. These are used to generate TEG tracings and which outline the viscoelastic change of the blood sample.
A number of cartridges are available and which allow various aspects of haemostasis to be analysed in real-time. For further information - see the Link to 'Haemonetics and TEG6s' system.
2a. ROTEM [ROTEM®]
The figure below shows the traces obtained using the ROTEM® device. The ROTEM analyser provides a trace similar to that of the TEG with related parameters including clotting time (CT) and maximum clot firmness (MCF). The table summarises the descriptive data reported with both the TEG® and ROTEM® devices.
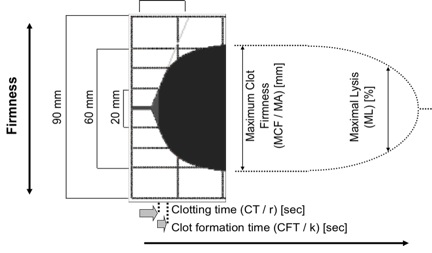
Variables measured by the TEG® and ROTEM® Devices
| Variable | TEG® | ROTEM® |
|---|---|---|
| Time [minutes] from the start of the test to when the waveform reaches 2mm above baseline. This represents the initiation of coagulation |
R | Clotting Time [CT] |
| Time [minutes] from 2mm above baseline to 20mm above baseline. This represents the amplification process of coagulation |
K | Clot Formation Time [CFT] |
| Alpha angle [°]. This represents the Thrombin burst |
The angle between the end of the R time and 20mm above the base line - the K time. | The angle between the end of the CT time and 20mm above the base line - the CFT time |
| Maximum strength [mm] - and which measures the maximum displacement of the waveform. This represents the overall strength of the clot and is dependent upon the formation of Fibrin, the interaction with platelets, stabilisation of the Fibrin clot by Factor XIII and the Haematocrit. |
Maximal Amplitude [MA] | Maximal Clot Firmness [MCF] |
| Time [minutes] to Maximum Clot Firmness | - | MCF-t |
| Amplitude [mm] at a specific time point after the start of the end of the R/CT times e.g. 5 minutes [A5], 10 minutes [A10], 30 minutes [A30], 60 minutes [A60] etc | A30, A60 | A5, A10... |
| Clot Elasticity [dyn/cm2] - measures the 'firmness' of the clot | E | CE |
| Maximum Lysis - a measure of the percent of the decrease in amplitude of the waveform at the end of the test . A ML value of <15% at 60 minutes indicates a stable clot whereas an ML value of >15% is indicative of fibrinolysis. |
- | ML |
| Clot Lysis at a specific time [minutes] - a measure of the percent of decrease in waveform amplitude at 30 and 60 minutes after the waveform has reached maximum amplitude [MA/MCF]. This measures clot stability and Fibrinolysis. |
CL30, CL60 | LY30, LY45, LY60 |
Modifications of the ROTEM® and TEG® Devices
The TEG® device originally used fresh non-anticoagulated whole blood but now both the TEG® and ROTEM® commonly employ citrated whole blood that is re-calcified to initiate coagulation. It is also common practice to use an activator as this standardises the test and in addition speeds up the rate at which clotting takes place and hence the rate at which a result is generated.
1.
Modifications of the TEG®
| Modification | Interpretation |
|---|---|
| Native TEG | No modifications but citrated whole blood is recalcified with CaCl2 to initiate coagulation |
| Tissue Factor Similar to the PT |
The use of an activator when undertaking Thromboelastography is generally recommended to standardise the initiation of the clotting process. Tissue Factor activation of the TEG enables the maximal amplitude (MA) to be established within 10 minutes but will result in significant shortening of the reaction time (R value) and as a result much of this latter information is lost. |
| Kaolin Similar to the APTT |
Contact activation. Isolates the intrinsic pathway of coagulation but will result in significant shortening of the reaction time (R value) and as a result much of this information is lost. |
| Heparinase and the R Time [HTEG] |
The R value in a native TEG is sensitive to trace amounts of Heparin and endogenously released Heparan sulphate. The use of a Heparinase-coated reaction cuvette for the TEG will demonstrate any Heparin present in the sample or in the patient and enables assessment of haemostasis in patients who are fully anticoagulated with Heparin e.g. on cardio-pulmonary bypass [CPB]. |
| Tissue Factor/Kaolin activated TEG and the ACT [rTEG or Rapid TEG] | By incorporating both Tissue Factor and Kaolin into the TEG cuvette, the TEG approximates to the Activated Clotting Time [ACT.] |
| Abciximab Fibrinogen and Platelet function |
The MA is primarily a reflection of clot strength and is affected by changes in Fibrinogen, the platelet count, platelet function and Factor XIII. The MA is established either from a native sample with no activator or from the combined Tissue Factor/Kaolin activated TEG.
There is a strong linear correlation between the log platelet count and MA. Abciximab is a potent platelet GpIIb/IIIa inhibitor and an Abciximab-modified TEG can help to discriminate between Hypofibrinogenaemia and Platelet dysfunction as a cause of a decreased MA. |
| Fibrinolysis | The degree of fibrinolysis can be established from either a native sample with no activator, from the Tissue Factor activator or the combined Tissue Factor/Kaolin activated TEG. Hyperfibrinolysis is increasingly recognised as a cause of peri-operative microvascular bleeding and is readily detected by analysing the clot lysis index on the TEG or ROTEM. The ability to detect and determine the severity of fibrinolysis avoids empirical or inappropriate anti-fibrinolytic therapy. Mathematical derivations of changes in elastic modulus derived from the amplitude have been used to quantify the extent of fibrinolysis in clinical and laboratory settings, as well as to guide antifibrinolytic therapy. |
| Hypercoagulability | The TEG may be helpful in screening for hypercoagulable states. TEG analysis of patients with a history of thromboembolic complications showed shorter R values and accelerated clot propagation compared to healthy reference subjects. |
| PlateletMapping TEG | The TEG PlateletMapping® assay provides a method for testing the ability of platelets to participate in clot formation with and without the contribution of anti-platelet drugs. The assay involves four separate analyses on whole blood: 1. A Kaolin-activated TEG which generates Thrombin to activate platelets and cleave Fibrinogen. This provides a measurement of overall clot strength. 2. A TEG in which heparin is used to inactivate Thrombin and Reptilase and Factor XIIIa are used generate a fibrin clot and to isolate the fibrin contribution to overall clot strength. 3. An ADP TEG in which platelets are activated using ADP. This TEG includes Reptilase and Factor XIIIa. 4. An Arachidonic Acid TEG which activate the platelets via the TxA2 pathway. This TEG includes Reptilase and Factor XIIIa. By comparing the relative clot strengths of the 4 assays, it is possible to determine the degree of platelet inhibition in the presence of defined anti-platelet agents. |
2. Modifications of the ROTEM®
| Test | Modification |
|---|---|
| INTEM Similar to the APTT |
Contains Phospholipid and Ellagic acid as activators and provides information similar to that of the APTT |
| EXTEM Similar to the PT |
Contains Tissue Factor as an activator and provides information similar to that of the PT |
| HEPTEM |
Contains lyophilised Heparinase for neutralising heparin. Coagulation is initiated as for the INTEM. |
| APTEM | Contains Aprotinin for inhibiting fibrinolysis. Coagulation is initiated as in the EXTEM. A comparison of the data from the EXTEM and the APTEM allows for the rapid detection of hyperfibrinolysis and in addition can determine if antifibrinolytic therapy alone will normalise coagulation or if additional treatments such as Fibrinogen supplementation, is necessary. |
| FIBTEM | Based upon the INTEM but in addition contains Cytochalasin D, a platelet inhibitor which blocks the platelet contribution to clot formation, allowing a qualitative analysis of the functional Fibrinogen component. |
| ECATEM | Contains Ecarin and so is similar to the Ecarin Clotting Time. This makes it very sensitive to presence of Direct Thrombin Inhibitors [DTIs]. |
| NATEM | Non-citrated whole blood with no additional reagents. This must be processed immediately after collection. |
2b. ROTEM® SIGMA
The ROTEM® SIGMA is a fully automated system based upon the ROTEM Thromboelastometry system and requires no pipetting of a blood sample or test preparation. In the ROTEM® SIGMA device the reagents for the tests are contained as freeze-dried pellets within a cartridge. The tests are performed by inserting a cartridge into the machine and attaching a blood sample to the analyser.
The ROTEM® SIGMA cartridges consist of five tests in two test combinations. The tests in each cartridge are performed simultaneously using the four channels of the analyser:
Cartridge 1: Cartridge 1 comprises 4 tests:
| FIBTEM C | EXTEM C | INTEM C | APTEM C |
| Extrinsic pathway activation with platelet inhibition. | Extrinsic pathway activation with Tissue Factor. | Intrinsic pathway activation with Ellagic Acid. | Extrinsic pathway activation with inhibition of Fibrinolysis. |
| Contains Tissue Factor as the activator and Cytochalasin D - an actin polymerization inhibitor and which blocks the contribution of platelets to clot formation. The FIBTEM C, therefore, assesses the role of Fibrinogen in clot formation as the contribution of platelets has been removed. | Assesses the Extrinsic pathway of coagulation including Fibrin polymerisation and Fibrinolysis. | Assesses the Intrinsic pathway of coagulation including Fibrin polymerisation and Fibrinolysis. | Contains Tissue Factor to activate the Extrinsic pathway of coagulation and Aprotinin to inhibit Fibrinolysis. |
PLTTEM: The PLTTEM is a parameter that is used to evaluate the contribution of platelets to clot firmness and is derived by subtracting the FIBTEM amplitude from the EXTEM amplitude. It can be calculated at various time points - see References.
Cartridge 2: Cartridge 2 comprises 4 tests:
| FIBTEM C | EXTEM C | INTEM C | HEPTEM C |
| Extrinsic pathway activation with platelet inhibition. | Extrinsic pathway activation with Tissue Factor. | Intrinsic pathway activation with Ellagic Acid. | Intrinsic pathway activation with inhibition of Heparin. |
| Contains Tissue Factor as the activator and Cytochalasin D - an actin polymerization inhibitor and which blocks the contribution of platelets to clot formation. The FIBTEM C, therefore, assesses the role of Fibrinogen to clot formation as the contribution of platelets has been removed. | Assesses the Extrinsic pathway of coagulation including Fibrin polymerisation and Fibrinolysis. | Assesses the Intrinsic pathway of coagulation including Fibrin polymerisation and Fibrinolysis. | Contains Ellagic acid as the activator and Heparinase for neutralising unfractionated Heparin. |
Interpretation of Results obtained from the ROTEM® and TEG® Devices
| --- Device --- | ||
|---|---|---|
| TEG® | ROTEM® | Indicative of... |
| Prolonged R Time | Prolonged Clotting Time [CT] | Factor deficiency Factor dysfunction Presence of anticoagulants Severe Hypofibrinogenaemia Severe thrombocytopenia |
| Prolonged K Time | Prolonged Clot Formation Time [CFT] | Factor deficiency Thrombocytopenia Platelet dysfunction Hypofibrinogenaemia |
| Decreased α angle | Decreased α angle | Thrombocytopenia Platelet dysfunction Hypofibrinogenaemia |
| Decreased Maximal Amplitude [MA] | Decreased Maximal Clot Firmness [MCF] | Thrombocytopenia Platelet dysfunction Hypofibrinogenaemia |
| Increased in EPL or LYS30/LYS60 | CLF | Primary fibrinolysis Secondary fibrinolysis |
Derived TEG Parameters
A number of parameters can be derived from the TEG and these include:
1. Thrombin Generation: The rate and amount of Thrombin generation is considered to be predictive of the risk of thrombosis or haemorrhage. The TEG system provides the 'Thrombus Generation Velocity Curve' [see References] - the first derivative of the standard TEG waveform. Although the graph appears similar to that of a Thrombin Generation Test, it provides different information. In addition to providing information on thrombus generation it can also provide similar information on the rate of lysis of the Fibrin clot.
Similar information can be generated using the ROTEM device.
2. Clotting or Coagulation Index [CI]: A global assessment of clot formation using a combination of the variables: R, K, MA and Alpha [α] angle.
The Coagulation Index (CI) for whole blood is calculated as follows:
...where R, K, MA and the α angle, are values generated from the TEG waveform.
Normal values for the Coagulation Index [CI] lie within -3.0 and +3.0, which is three standard deviations from the mean of zero. A Positive value [CI > +3.0] indicates the sample is hypercoagulable whilst a Negative value [CI < -3.0] indicates that the sample is hypocoagulable.
This is illustrated in the table below:
| TEG | R Time [min] | K Time [min] | MA [mm] | Alpha Angle | Coagulation Index [CI] |
|---|---|---|---|---|---|
| Normal | 6 | 2 | 63 | 60 | 2.52 |
| Hypocoagulable | 12 | 8 | 30 | 32 | -3.63 |
| Hypercoagulable | 3 | 3 | 86 | 91 | 6.34 |
3.
Shear Modulus Strength [G]: Measures the firmness or strength of the clot as it is generated, measured in dyn/cm2 or kdyn/cm2.
It is derived from the Amplitude [A] of the clot using the formula:
G = (5000 x A)/(100-A)
and is generated continuously.
The Shear Modulus Strength [G] calculated at the Maximum Amplitude [MA] in the TEG or at the Maximum Clot Firmness [MCF] in the ROTEM, yields the maximum strength of the clot. If, for example the MA in the TEG was 50mm then the G value is:
G =
(5000 x 50)/(100-50) = 5000
dyn/cm2 (or 5 kdyn/cm2).
A normal range for the G value in a healthy individual using a RAPID TEG is in the region of 5300 - 12400 dyn/cm2. An elevated G value indicates a potentially increased risk of thrombosis and a
reduced G value indicates reduced clot strength and potentially an increased risk of bleeding.
4.
Elasticity Constant [E in the TEG; CE in the ROTEM]: This is the normalised value of G in the TEG or the ROTEM and is derived from the formula:
E or CE = (100 x A)/(100-A) expressed in dyn/cm2
where A is the amplitude of the clot. This Elasticity Constant is calculated continuously. A normal MA of 50mm will yield an E value of 100 dyn/cm2.
[There is debate as to whether the value for E or CE should be dimensionless i.e. omit dyn/cm2.]
4. Thrombodynamic Potential Index [TPI]: This is the Elasticity Constant [E] obtained at the
Maximum Amplitude [MA] divided by the Kinetics Time [K] measured in mm.
TPI = [(MA x 100)/(100–MA)]/K
When the TPI <6 this represents a hypocoagulable state, whereas a TPI >7.5 represents a hypercoagulable state.
6. Clot Lysis Index [CL30, CL60]:
A measure of clot lysis derived from the relationship of the amplitude of the clot at 30 minutes [A30] or 60 minutes [A60] to the Maximum Amplitude [MA] of the clot:
CL30 = 100 x (A30/MA)
CL60 = 100 x (A60/MA)
7. Estimated Percent Lysis [EPL]: A computer prediction of the estimated rate of change in amplitude after the MA is reached. It may be the earliest indicator of abnormal lysis as it can begin 30s after the MA has been reached.
8. Thrombodynamic Ratio [TDR or TR]: This is a measure of hypercoagulability and derived from the formula:
TDR or TR = [MA x Tan(α)]/R
where MA is the Maximum Amplitude, α is the alpha angle and R is the Reaction Rate. There are no units associated with the TDR.
In a healthy individual if the MA is 50mm, the alpha angle 54° and the R time 7.6 minutes then:
TDR or TR = [50 x Tan(54)/7.6 = [50 x 1.38]/7.6 = 9.07
Increased TDR values are associated with an increase in hypercoagulability. See Artang et al 2000 for additional information.
Interpretation
See references for further information on the interpretation of the various traces generated by the TEG® and ROTEM®.
Reference Ranges
It is important to remember that the shape of the waveform is often as useful as the results of individual values. Reference ranges will also change depending upon whether a test uses an activator [e.g. Tissue Factor or not i.e. a native blood sample], the age of the patient and in the case of a female, if pregnant. Reference ranges for healthy individuals should be established locally. Published data for in the paediatric setting have been published - see references.
EQA Schemes
Internal Quality Control [IQC] and External Quality Assurance [EQA] are a fundamental part of any test that a laboratory offers and this includes viscoelastic haemostatic assays. NEQAS BC in the UK provides an EQA scheme for viscoelastic haemostatic assays - see LINKS.
Thromboelastography is increasingly used as a Point of Care Test [POCT] and it is essential that the staff using these machines in this setting are appropriately trained and in addition participate in appropriate EQA schemes to ensure the accuracy and validity of the results which may guide clinical practice.
Emerging Technologies
A number of novel methods for point-of-care viscoelastic haemostatic assays are in development and these include:
- Laser Speckle Rheometry
- Mechanical Resonant Frequency
- Ultrasonic Deformation
- Parallel Plate Viscometry
- Traditional Viscoelastic Testing with 'Active Tips'
...for more information - see Hartmann et al 2020; Volod et al 2022..
What Test Next
On the basis of the TE traces, additional tests may be suggested. However, the TEG® and ROTEM® machines are often used to both investigate disorders of haemostasis and guide blood product replacement without additional tests and they may, therefore, be performed in isolation.
Click HERE to return to the top of the page.
