Light Transmission Aggregometry [LTA]
Introduction
Platelets are fundamental to primary haemostasis and the different functions can be investigated using a variety of methodologies. Platelet function testing is time consuming and prone to a wide-variety of problems due to pre-analytical variables. Light Transmission Aggregometry [LTA] is frequently undertaken as the first test of platelet function, as a screening test for a bleeding disorder and in addition for monitoring of anti-platelet drugs.
Before undertaking any tests of platelet function - consider:
| Variable | Interpretation |
|---|---|
| Clinical History & examination. | Some syndromes [e.g. Hermansky Pudlak syndrome, Cheddiak Higashi syndrome, Wiskott-Aldrich syndrome, Velocardiofacial Syndrome (VCFS), Noonan syndrome, MYH9-related disorders] are associated with abnormal platelet function and you may get some idea of the diagnosis from the clinical history and examination. |
| Drug History | There are a large number of drugs and food substances that can interfere with platelet function. |
| Full Blood Count (FBC) and Blood Film | 1. Pseudo-thrombocytopaenia: often due to cold reacting platelet agglutinins or to platelet satellitism. Approximately 0.1% of the healthy population show EDTA-induced Pseudo-thrombocytopaenia and it is important to exclude this before undertaking more extensive tests of platelet function. Similar findings have also been reported with the use of both citrate and heparin as anticoagulants. A blood film may identify platelet clumps and provide a clue to the diagnosis. 2. Mean Platelet Volume: [MPV – reference range 7-10fL]: The MPV is an often ignored parameter of the FBC but can provide important insights into the causes of a low platelet count. - It can also in some cases give a clue to the diagnosis e.g. the hereditary macrothrombocytopenias, Bernard Soulier Syndrome [BSS] - In individuals with an elevated MPV, an immunological-based platelet count may provide a more accurate and often significantly higher platelet count. - The MPV can be an indication of platelet turnover – an increased MPV indicating accelerated platelet clearance as in ITP or gestational thrombocytopaenia. - The MPV may be reduced in cases of Wiskott-Aldrich Syndrome and in some cases of bone marrow failure. 3. Blood Film and Platelet Morphology: An examination of the blood film and platelet morphology can be useful in both establishing a diagnosis of Pseudo-thrombocytopaenia but also in establishing a primary platelet problem e.g. Gray Platelet Syndrome. In some cases of thrombocytopaenia e.g. May Hegglin anomaly – the blood film may show the presence of Döhle bodies [light blue-gray, oval, basophilic, leukocyte inclusions located in the peripheral cytoplasm of neutrophils.] |
Principles of Light Transmission [Born] Aggregometry
Platelet aggregation testing measures the ability of various agonists to platelets to induce in vitro activation and platelet-to-platelet activation. Classically Born aggregometry uses Platelet Rich Plasma [PRP] but whole blood aggregometry can be also used.
In the Born aggregometer, PRP is stirred in a cuvette at 37°C and the cuvette sits between a light source and a photocell. When an agonist is added the platelets aggregate and absorb less light and so the transmission increases and this is detected by the photocell.
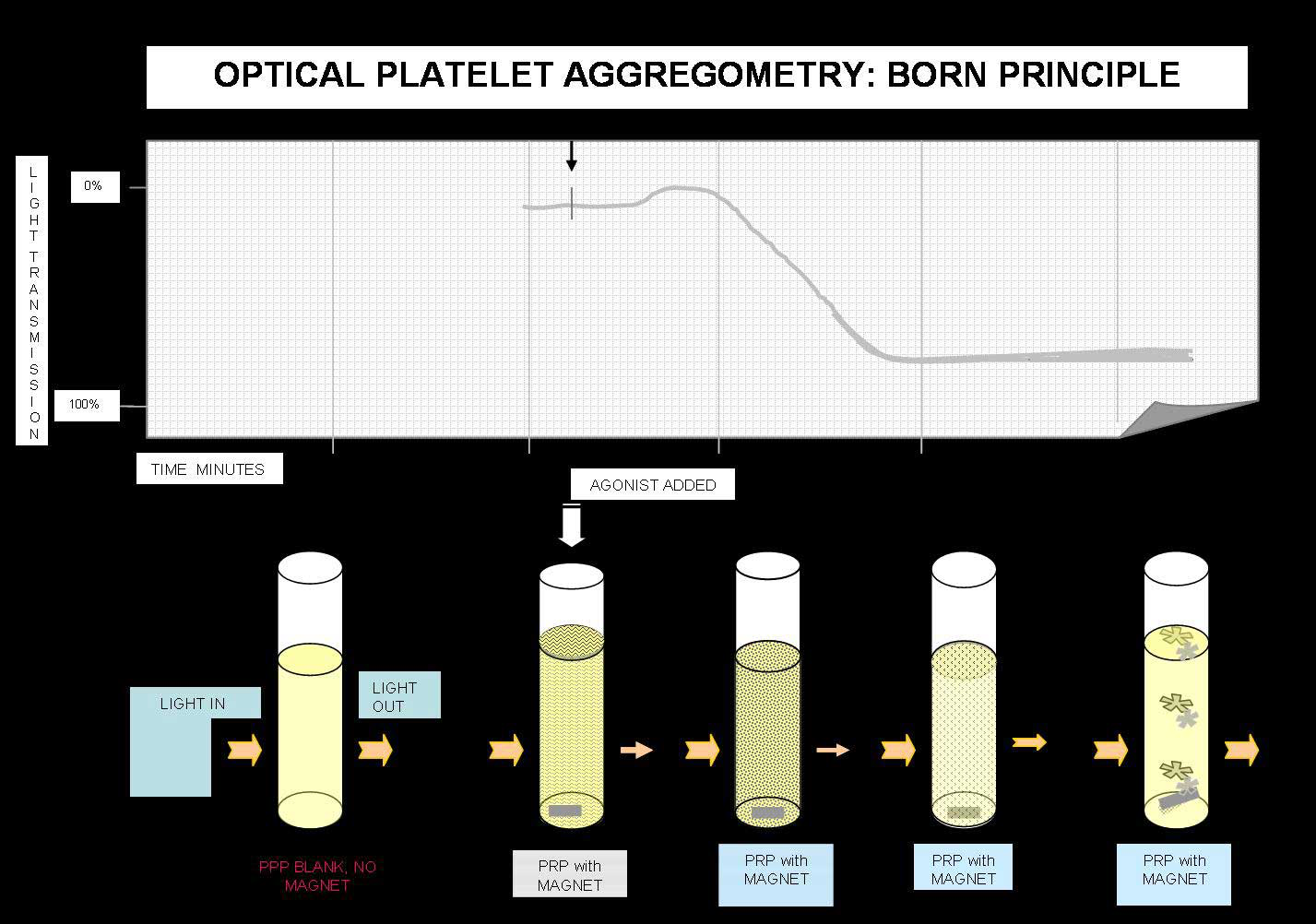
Light Transmission Aggregometry: Variables
| Variable | Explanation |
|---|---|
| Pre-Analytical Variables | Drugs: Drugs which can interfere with platelet function include aspirin and anti-inflammatory drugs, specific anti-platelet drugs including clopidogrel and imidazole. However, there are numerous other drugs whose primary role is not to inhibit platelet function but nevertheless can do so e.g. antibiotics, anti-depressants, beta-blockers etc. Food stuffs - A high fat diet can lead to the presence of chylomicra in the plasma and interfere with light transmission in aggregation testing. - Others include garlic, turmeric and caffeine. Platelet count: In individuals with very high or low platelet counts, it may be necessary to adjust the platelet count to achieve a count in the region of 200-400 x 109/L. For very high counts the count can be adjusted with PPP. Platelet counts below 200 x 109/L can give rise to diminished aggregation responses. Although it seems logical to undertake additional centrifugation in such cases to increase the platelet count, in practice this can lead to activation of platelets and is not recommended. Temperature: Blood samples for platelet aggregation testing should be stored at room temperature. pH: Platelet aggregation should be carried out at physiological pH. Fibrinogen Concentration: Platelets will only aggregate (although they may agglutinate) if fibrinogen is present and so it is important to check fibrinogen levels before undertaking platelet aggregation testing. Anticoagulant: Current guidelines suggest that samples for platelet aggregation testing should be collected into citrate. However, more recent data suggests that heparin, but not citrate, preserves platelet responses for up to 24 h as determined by a range of techniques. |
| Preparation of Platelet Rich Plasma [PRP] |
Anticoagulant: Venous blood with minimal venous occlusion, is collected into 3.2%/0.109M citrate in a ratio of 1:9 [1 part anticoagulant to 9 parts blood.] Whole blood samples should be processed within 4 hours of collection. Blood samples for platelet aggregation testing should be stored at room temperature – cooling platelets can lead to activation. Transport samples to the laboratory at room temperature. PRP is prepared by centrifugation at 20°C for 10-15 minutes at 150-200g. The PRP is carefully removed and placed into a stoppered plastic tube. PRP should be stored at room temperature. PPP can be prepared by further centrifugation of the remaining plasma at 2700g for 15 minutes. |
| Agonists | Addition of a platelet agonist to the PRP leads to platelet activation, a change in their shape from discoid to spiny spheres which is associated with a transient increase in optical density. The only exceptions to this are Epinephrine in which there is no shape change and Ristocetin which causes platelet agglutination rather than aggregation i.e. there is no binding of fibrinogen. There are two types of agonists: Strong Agonists e.g. Collagen, Thrombin, TxA2: These directly induce platelet aggregation, TxA2 synthesis and platelet granule secretion. Weak Agonists e.g. ADP & Epinephrine: These induce platelet aggregation without inducing secretion. Platelet secretion can sometimes follow aggregation induced by a weak agonist, when the synthesis of endogenous TxA2 is triggered by the close platelet-to-platelet contact that occurs during platelet aggregation. Strong agonists, when used at low concentrations, may act like weak agonists, but weak agonists even at high concentrations will not act as strong agonists. With some weak agonists [ADP and Epinephrine] at critical concentrations, the platelet aggregation curve has a biphasic appearance: an initial wave of aggregation (primary wave), followed by a secondary wave of aggregation, which is usually irreversible [see illustration below.] Secondary wave aggregation may not occur and the primary wave may disaggregate. At higher agonist concentrations (except with epinephrine) the two waves of aggregation combine and only a single wave is seen and the biphasic waveform is absent. The aggregation response to an agonist is amplified by the production of TxA2 from membrane phospholipids and by the secretion of ADP from the dense granules. ADP and TxA2 are agonists, which, by interacting with their specific receptors, amplify the aggregation response of the platelet. |
Commonly used Agonists in Light Transmission Aggregometry
Commonly used agonists, their working concentration and mode of action are listed below. In practice many laboratories use a number of agonists and various dilutions but vary the actual agonists or agonist concentration depending upon the results of initial tests and the suspected abnormality. Not all laboratories necessarily use the concentrations shown below e.g. some labs may use Collagen at 5μg/mL rather than 4μg/mL.
It is useful to consider agonists together with an image of the various receptors on the surface of the platelet - see Ref 24 for example.
| Agonist | Working Concentration | Comment |
|---|---|---|
| ADP | Low dose: 1, 2.5, 5μM High dose: 10μM |
ADP binds to the ADP receptor on the surface of platelets. Initial binding results in the release of intracellular calcium and a change in the shape of the platelet leading to the primary wave of aggregation. The secondary wave reflects the release of ADP from platelet storage granules. Low dose ADP induces only primary aggregation and the effect is reversible. ADP and Arachidonic acid are considered mild platelet agonists. ADP binds to two G-protein coupled receptors: P2Y1 and P2Y12. Binding of ADP to the P2Y1 receptor induces shape change and initiates primary wave platelet aggregation through calcium mobilisation. The P2Y12 receptor is considered to be the major ADP receptor and responsible for full platelet aggregation through the inhibition of adenyl cyclase. The P2Y12 receptor is also the target for clopidogrel. With both ADP and Arachidonic acid - this second wave of aggregation is inhibited by aspirin and NSAID's. |
| Collagen | 1, 4μg/mL | Collagen binds to the GpVI and GpIa/IIa receptors on the surface of the platelet, inducing granule release, TXA2 generation and then sustained GPIIb-IIIa activation. The GpIa/IIa receptor is involved in platelet adhesion. The GpVI receptor is involved in platelet signalling and TXA2 generation. A lag phase is seen with collagen following addition of the agonist to the PRP and usually <1 minute. A low concentration of Collagen is used to detect impaired platelet function from Aspirin and other COX-1 inhibitors. A higher concentration of Collagen should be used if the low concentration demonstrates impaired aggregation. |
| Ristocetin | Low dose: 0.5mg/mL High dose: 1.5, 5mg/mL |
Ristocetin (but not generally in low dose i.e. 0.5 mg/mL) causes platelet agglutination (and not aggregation) through the VWF and GPIb-IX-V complex. Platelet aggregation requires the binding of fibrinogen to the platelet via the GpIIb-IIIa complex. |
| Adrenaline | 5, 10μM | Adrenaline binds to the α2-adrenergic receptor on the surface of platelets leading to inhibition of adenyl cyclase and the release of calcium ions. Aggregation of platelets with Adrenaline is similar to that of ADP with an initial primary wave of aggregation, the release of stored ADP from the platelet dense bodies and second wave sustained aggregation. As with ADP, this second wave of aggregation is inhibited by Aspirin and NSAIDs. Adrenaline is considered [as is ADP] to be a weak agonist.
However, defects in signalling through the α2-adrenergic have been associated with a bleeding disorder. A small proportion of the population may not always show full aggregation to Adrenaline due to natural variations in adrenoreceptor numbers. Such individuals do not have a platelet defect. |
| Arachidonic Acid | 500μg/mL | Arachidonic Acid is the precursor of Thromboxane A2 [TXA2] within platelets. Arachidonic Acid is converted to TXA2 by cyclooxygenase and Thromboxane synthase. TXA2 is a potent inducer of platelet aggregation causing granule release, more TXA2 generation and then sustained GpIIb-IIIa activation |
| Thrombin | Low dose: 50nmol/L High dose: 100nmol/L |
Thrombin is the most potent physiological activator of platelets and Protease-Activated Receptors 1 (PAR1) and 4 (PAR4) are activated by Thrombin. PAR1 and PAR4 are members of a 7-transmembrane group of G-protein coupled receptors that are activated by a single cleavage within the N-terminal domain to generate a new N-terminus which activates the G-subunits and intracellular signalling. Thrombin induces platelet aggregation at low concentration [50nmol/L] but at this dose it is insufficient to induce full aggregation but binds to platelets and induces a shape change. At a higher concentration (100nmol/L), Thrombin induces full aggregation. |
| Thromboxane A2 [TxA2] analogue U46619 | 1nM | U46619 is a potent and stable TxA2 agonist. |
Method
Platelet aggregometry is performed as follows:
| Step | |
|---|---|
| 1 | Platelet aggregometry is performed at 37°C. |
| 2 | The aggregometer is calibrated by: - A cuvette containing PRP which equates to 0% light transmission - A second cuvette containing PPP which equates to 100% light transmission. |
| 3 | Platelets will only aggregate if they are activated (with an agonist) and in contact with each other - so they must be stirred whilst testing is taking place. Absence of stirring will lead to an absence of, at least a significant reduction in, aggregation. A check for spontaneous platelet aggregation [SPA] is made. SPA is rare in healthy individuals but seen in some cases of VWD, in some patients with diabetes, in some lipid disorders and in a variety of other disorders and is screened for in a sample by placing undiluted PRP in the aggregometer and stirring for 15 minutes. In cases of SPA, dilution of the PRP may abolish this and if the platelet count remains >200 x 109/L then aggregation testing can proceed. |
| 4 | In general - 270μL of PRP is added to the aggregometry cuvette and warmed at 37°C until a steady baseline is achieved. 30μL of the agonist is added the response recorded. The tests are repeated using a panel of agonists. |
The following aggregation trace shows the events in classic biphasic aggregation:
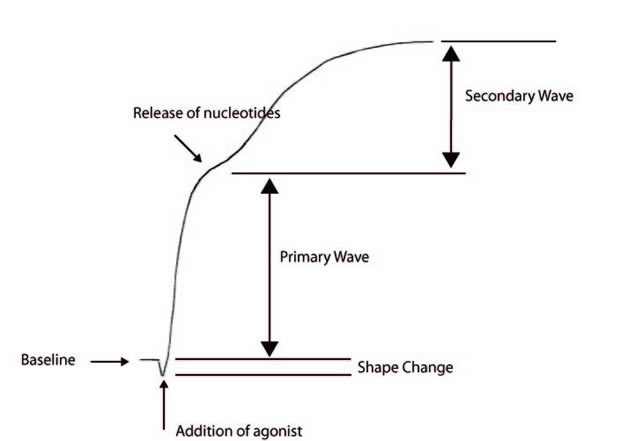
1. Baseline
2. Addition of an Agonist - this results in a change in platelet change and hence a drop in the baseline absorbance
3. Primary wave aggregation
4. Release of nucleotides
5. Secondary wave aggregation
Adrenaline and low dose ADP classically give a biphasic aggregation curve whereas with a number of other agonists only a single wave is seen and it is not possible to distinguish the primary wave from the secondary wave.
Interpretation;
Calculating the slope or the rate of aggregation
Look at the image below:
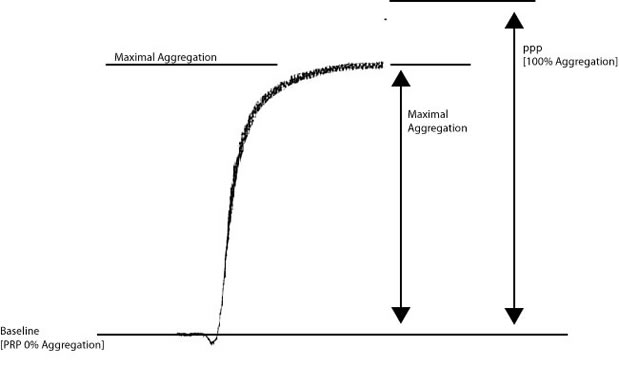
Historically, percentage [%] maximal aggregation has been reported when analysing aggregation curves. To calculate the % maximal aggregation, the distance between the baseline [0% aggregation - platelet rich plasma] and platelet poor plasma [100% aggregation] [Y] is divided by the maximal aggregation [X]. So in the example above if the Y = 100mm and X = 87mm then percentage maximal aggregation = X/Y = 87%.
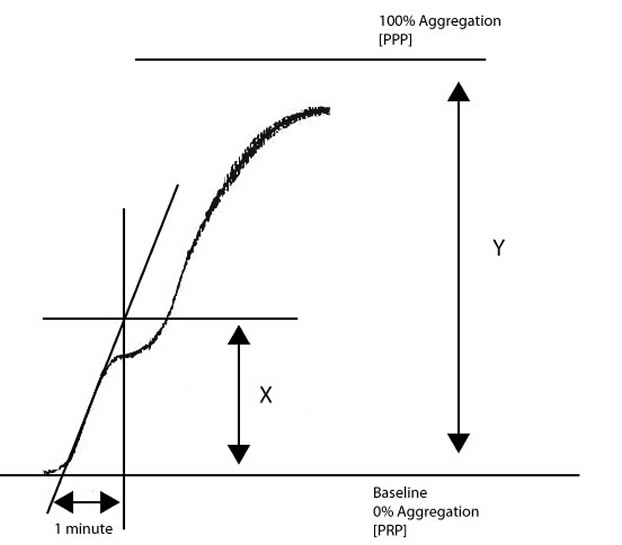
To calculate the slope [and this forms the basis of the VWF:RCo platelet-based functional assay]:
1. Draw
a line at a tangent to the aggregation curve.
2. Determine how many millimetres [mm] the chart recorder records in 1 minute.
3. Measure in mm from the point where the tangent intersects the baseline to the distance equal to 1 minute.
4. Draw a line perpendicular to the baseline from the ‘1 minute’ point to the intersect point of the tangent.
5. Measure the distance [in mm] covered from the baseline to the intersect point [X].
6. Derive the maximal height of the aggregation [100% aggregation or maximal aggregation] from the y-axis [Y].
Divide X/Y to calculate the slope or rate of aggregation.
In the example above, if X = 23mm and Y = 97mm, the slope is X/Y = 0.24
Interpretation of Platelet Aggregation Traces
The interpretation of platelet aggregation traces can be difficult. The attached file [click HERE] provides a summary of the abnormalities that may be identified by platelet aggregation testing.
Common aggregation traces that you are likely to encounter in an an exam-type setting are:
- Glanzmann's Thrombasthenia [or Afibrinogenaemia]
- Bernard-Soulier Syndrome [or Von Willebrand Disease]
- Storage Pool Disorder [or a release defect]
- The effects of Aspirin [or an aspirin-like defect]
- The effects of Aspirin + Clopidogrel
1. In the patient shown below, the only abnormality is a lack of agglutination with Ristocetin. Possible diagnoses are therefore, Von Willebrand Disease or Bernard Soulier Syndrome.
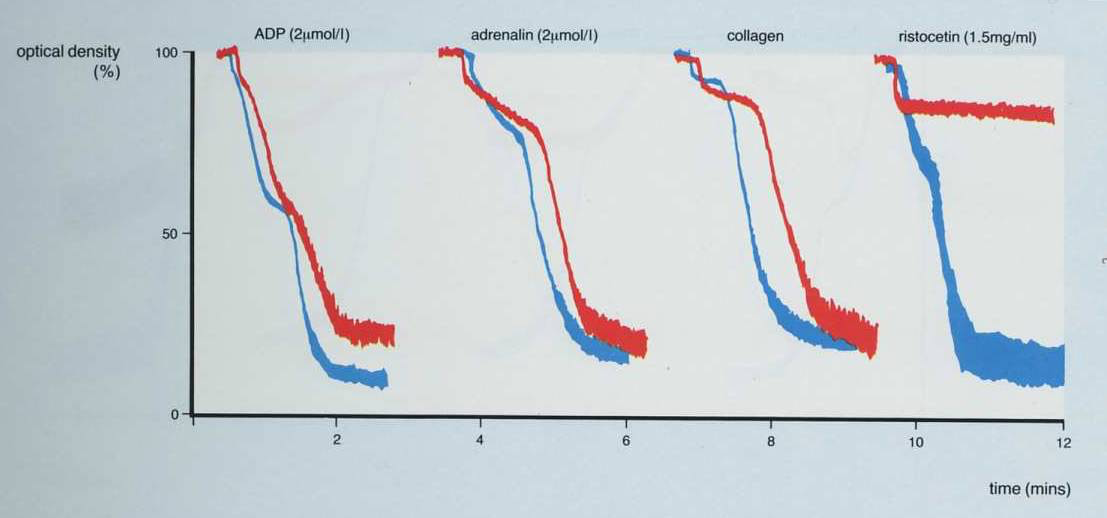
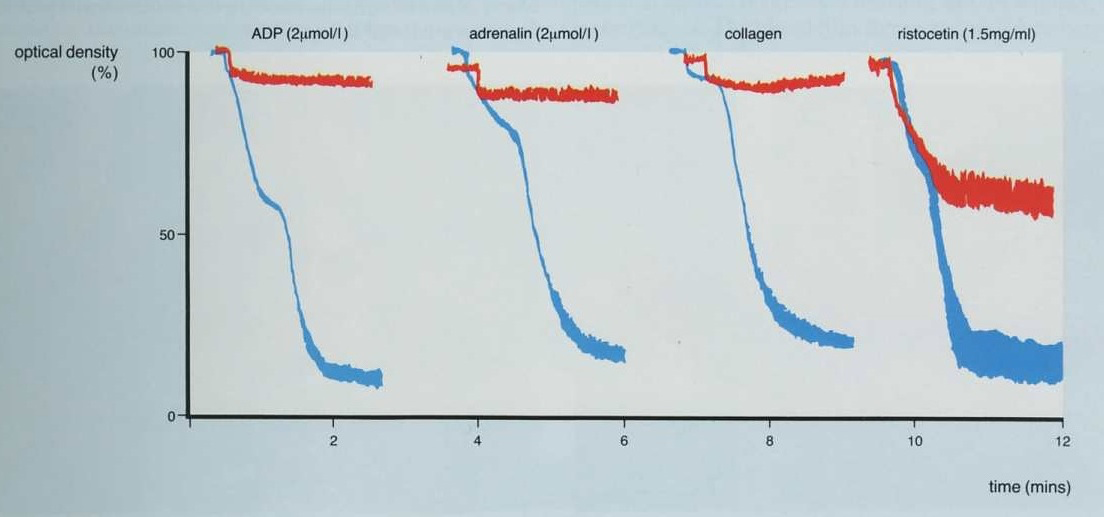
2. This is the converse of the patient shown above and the only agglutination [and this is not complete] is seen with Ristocetin. There is no aggregation with ADP, adrenaline or collagen.
Possible diagnoses include Glanzmann's Thrombasthenia or Afibrinogenaemia.
[
Remember, platelet agglutination with Ristocetin occurs independently of Fibrinogen.]
In the traces shown below it is clear that only partial agglutination is seen with Ristocetin emphasising that for aggregation to occur, binding of Fibrinogen to the GpIIb/IIIa receptor is necessary.
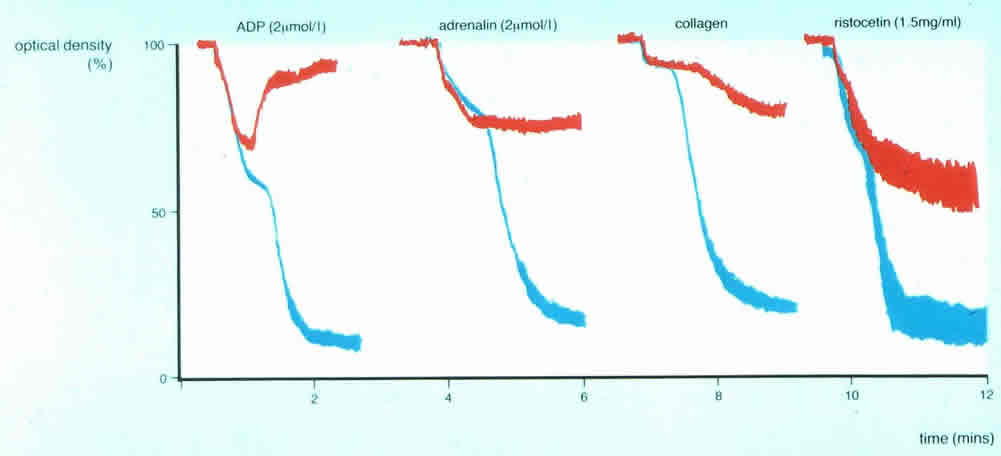
3. In this patient reversible, first wave aggregation is seen with ADP, adrenaline and collagen and only partial agglutination with Ristocetin. The picture is clearly different from the two traces above 1) or 2): the results suggest a failure of granule release and is consistent with either platelet Storage Pool Disorder or a defect in nucleotide release.
It is useful to summarise the 'commonly' described abnormalities seen with light transmission aggregometry [LTA] although in practice many of these are extremely rare. The table below summarises these:
| Disorder | Characteristic Findings on LTA |
|---|---|
| Glanzmann's Thrombasthenia OR Afibrinogenaemia |
Absent or markedly impaired aggregation to all agonists except Ristocetin. Ristocetin-induced agglutination shows only primary wave - aggregation cannot occur because fibrinogen cannot bind. Afibrinogenaemia gives similar results. |
| Bernard Soulier Syndrome OR Von Willebrand Disease | Absent or markedly reduced platelet agglutination with Ristocetin. |
| Storage Pool Disorder OR Platelet Release Defect | Primary aggregation only with ADP, adrenaline and collagen and only partial agglutination with Ristocetin suggesting a failure of granule release or a deficiency of platelet granules. |
| Aspirin [or defects in the COX pathway] | Absent aggregation to Arachidonic acid. Primary wave aggregation only with ADP. Decreased or absent aggregation with collagen. |
| Clopidogrel | Absent aggregation with ADP |
| 2B VWD/Platelet-type [pseudo]VWD | Agglutination with low dose Ristocetin e.g. 0.5 mg/mL. |
What test next?
On the basis of an abnormal platelet aggregation trace, you should establish if this fits in with any recognisable disorder. All abnormal results should be repeated and you may wish to undertake flow cytometry and nucleotide studies. Mutational analysis is now readily available to investigate abnormal platelet function in more detail.
A family pedigree should be constructed in all cases of a suspected platelet disorder - some of the rare platelet disorders are commoner in consanguineous relationships.
