Plasminogen Activator Inhibitor Type 1 [PAI-1]: Serpin E1 - Assays
Introduction
Plasminogen Activator Inhibitor Type 1 (PAI-1) also known as Serpin E1, is a serine protease inhibitor [SERPIN], secreted largely by endothelial and adipose cells and the major inhibitor of t-PA (tissue plasminogen activator) and u-PA [Urokinase]. PAI-1 is a single chain glycoprotein of MW 48KDa and consists of 379 amino acids. PAI-1 is stored primarily in platelets although is synthesised by a number of cells. Once released into the circulation it binds to t-PA or Vitronectin - the latter is able to stabilise and convert PAI-1 into an active form.
PAI-1 also plays a role in extracellular matrix remodelling including angiogenesis through its regulation of Plasminogen. PAI-1 levels may correlate with prognosis in certain forms of cancer, probably because of this role. PAI-1 levels may also be a risk factor for cardiovascular disease and post-operative thrombosis risk. Currently, however, PAI-1 levels do not have clear clinical relevance – i.e. they do not form the basis of therapeutic decision making or useful prognostic information in routine clinical practice.
During pregnancy Plasminogen Activator Inhibitor Type 2 (PAI-2) is secreted by the placenta with the same function as PAI-1.
The gene for PAI-1 [SERPINE1] maps to the long arm of Chromosome 7: 7q22.1. A sequence polymorphism [4G/5G] in the promoter region of the SERPINE1 gene at position -675 has been shown to affect PAI-1 levels and individuals who are homozygous for the 4G sequence tend to have the highest PAI-1 levels in contrast to 5G homozyous individuals who have the lowest.
A number of variables in addition to the 4G/5G polymorphism have been shown to influence PAI-1 levels and these include: BMI, Insulin resistance, various cytokines and hormones. Gram negative organisms have also been show to affect PIA-1 levels. See
Cesari et al - Ref 8 for an excellent review of the role of PAI-1 in disease.
A complete deficiency of PAI-1 is a rare autosomaly inherited disorder but is associated with an increased risk of bleeding due to accelerated fibrinolysis. It has been well studied in a large family belonging to the Old Order Amish population of Eastern and Southern Indiana. Affected females may have excessive bleeding associated with menstruation [menorrhagia] and abnormal bleeding in pregnancy and at delivery.
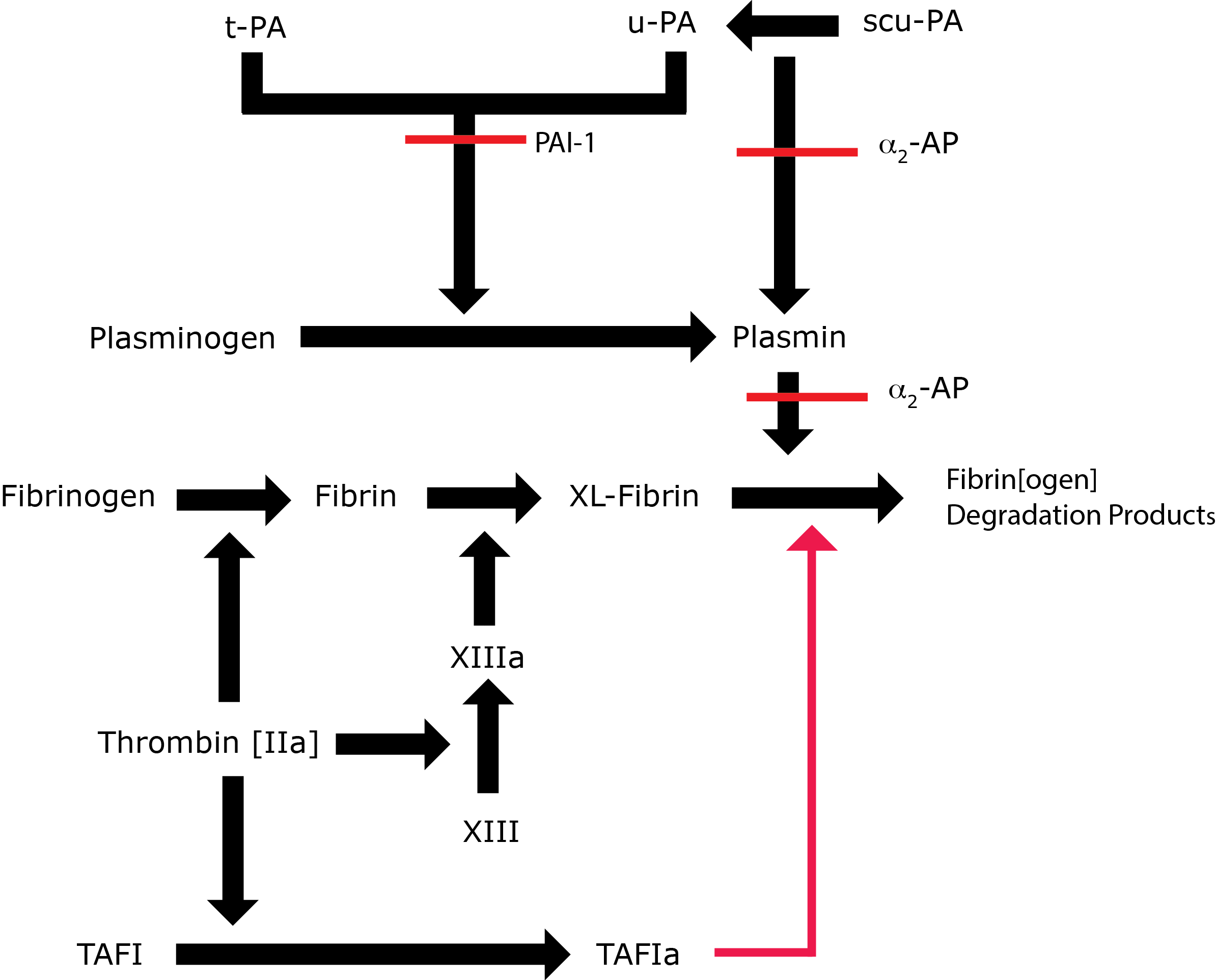
Key: t-PA - Tissue Plasminogen Activator; u-PA - Urokinase-type Plasminogen Activator; XL-Fibrin - cross-linked fibrin; TAFI - Thrombin Activatable Fibrinolytic Inhibitor; TAFIa - Activated Thrombin Activatable Fibrinolytic Inhibitor; α2-AP - Alpha-2-antiplasmin [Alpha2-Plasmin Inhibitor]; scu-PA - single chain Urokinase-type Plasminogen Activator. A red line indicates an inhibitory pathway.
Principles & Methods
Laboratory evaluation of PAI-1 is complicated by its role as an acute phase reactant and its’ interaction with t-PA, levels of which may also fluctuate under a wide variety of conditions.
| Assay | Methodology |
|---|---|
| ELISA | The ELISA assay has the advantage of being unaffected by t-PA:PAI-1 complex formation. A commonly employed ELISA assay involves the immobilisation of functionally active t-PA to microtitre plates using a monoclonal antibody. PAI-1 contained in the test sample binds to the immobilised t-PA and is then quantified using a peroxidase-labelled monoclonal anti-PAI-1 antibody. Many of these assays are specific for PAI-1 and will not detect PAI-2 e.g. in pregnancy. |
| Functional Assays | These rely on measuring the amount of residual t-PA or Urokinase activity in a sample containing a known excess of t-PA or Urokinase to which the patient’s plasma has been added. The most common methods use a chromogen to quantitate residual t-PA activity. 1. Latex Agglutination: An automated latex agglutination immunoassay (LIA) has also been described. Latex microparticles coated with an anti-PAI-1 antibody are mixed with patient plasma and agglutination (which is proportional to the amount of PAI-1 antigen present) measured photometrically. 2. Bioimmunoassay [BIA]: A micro-plate is coated with t-PA The patient sample is incubated with this and any PAI-1 present binds to the immobilised t-PA After washing a solution containing enzyme bound anti-PAI-1 antibody is incubated with the sample. After washing the enzyme substrate is incubated with the sample and the activity measured (e.g. by a chromogenic method). The amount of enzyme activity detected is proportional to the concentration of PAI-1 in the patient sample. The BIA for t-PA can be performed at acid pH to eliminate PAI-1 interference then again at alkaline pH to permit PAI-1 interference. The ratio of measured t-PA activity reflects the amount of PAI-1 present. 3. Chromogenic assay: This is a two-stage process. In the first stage, a known excess quantity of t-PA is incubated with patient plasma. This permits PAI-1:t-PA complexes to form. In the second stage a t-PA stimulator such as poly-D-lysine is added and the sample incubated with a chromogenic substrate specific for Plasmin. The colour change is proportional to the amount of t-PA activity in the sample which is, in turn, proportional to the PAI-1 activity in the test sample. In producing a standard curve for calibration of the bioimmunoassay and functional chromogenic assays, pooled human plasma and not recombinant t-PA should not be used as the latter does not give calibration curves suitable for assaying human t-PA A PAI-1 chromogenic detection method using venom from the Bitis arietans viper to eliminate the plasmin inhibitors α2-antiplasmin and α2-macroglobulin has been described. Plasma contains a number of anti-plasmins e.g. α2-AP and α2-macroglobulin and these require to be removed from the plasma before a 'true' functional PAI-1 assay can be undertaken. |
Interpretation
ELISA Assays:
These provide a very reliable quantification of total PAI-1 antigen
Functional assays:
1.
Bioimmunoassay: This measures PAI-1 activity in the sample but is less subject to interference from other variables than the standard chromogenic plasmin generation method. This is the most widely used functional assay.
2.
Plasmin generation chromogenic assay: The presence of agents which lyse Plasminogen will interfere with the test. Heparin binds to t-PA and increases its activity. Agents interfering with Plasmin may also interfere with the result.
These factors must be considered when interpreting results.
Abnormal tests should always be verified by repeat and abnormal levels should not be diagnosed on the basis of a single abnormal result.
Reference Ranges
Local normal ranges should be determined.
PAI-1 antigen 5-40 ng/ml
PAI-1activity <15 U/ml
What Test Next
Consider sequence analysis of the relevant genes if disordered fibrinolysis is suspected. In some cases Thromboelastography has proved very useful in monitoring fibrinolysis.
