Introduction
Fibrinolysis is the process by which Fibrin is removed from damaged blood vessels. Fibrinolysis is also important in tissue remodelling/repair after injury and in tumour metastasis.
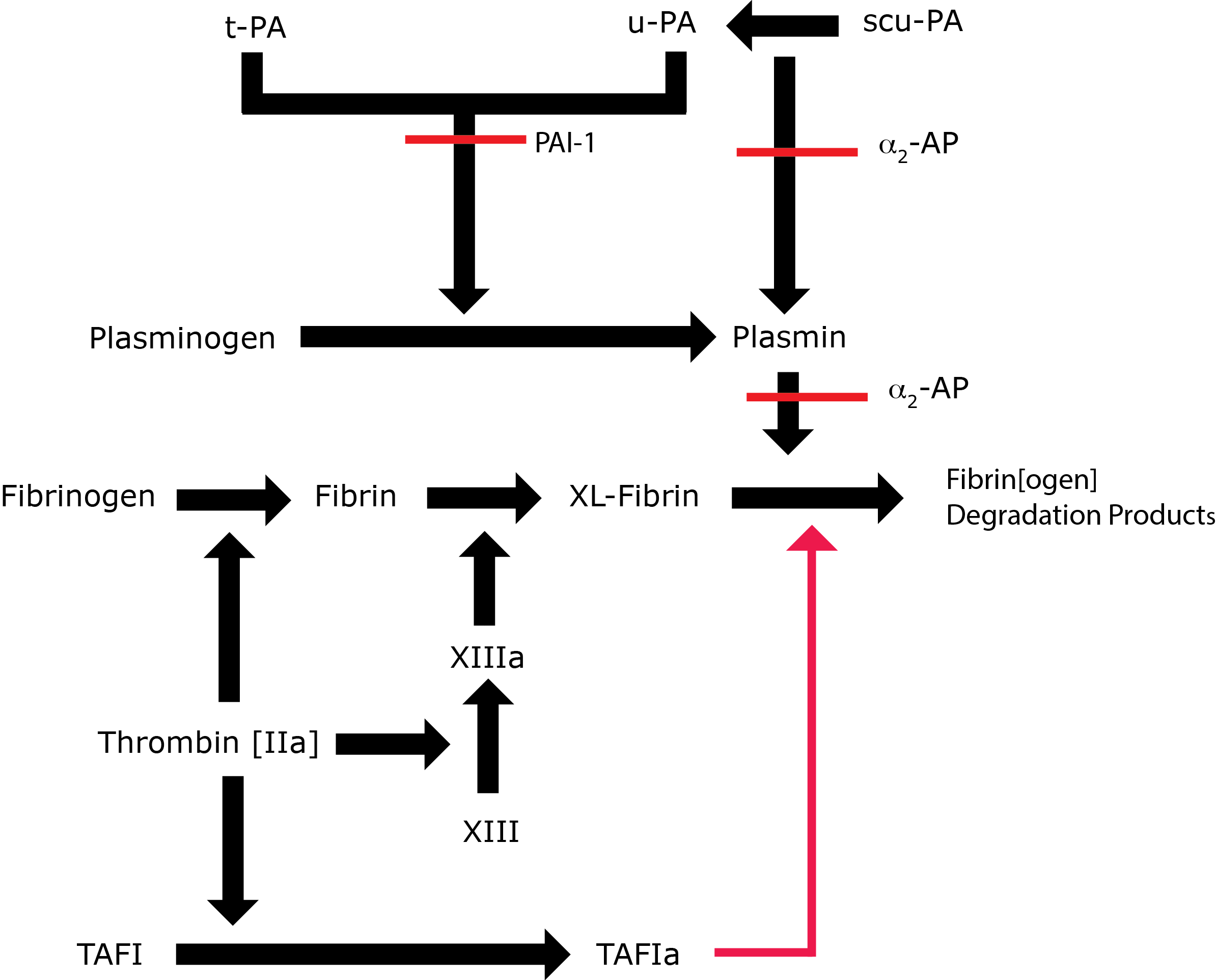
Key: t-PA - Tissue Plasminogen Activator; u-PA - Urokinase-type Plasminogen Activator; XL-Fibrin - cross-linked fibrin; TAFI - Thrombin Activatable Fibrinolytic Inhibitor; TAFIa - Activated Thrombin Activatable Fibrinolytic Inhibitor; α2-AP - Alpha-2-antiplasmin [Alpha2-Plasmin Inhibitor]; scu-PA - single chain Urokinase-type Plasminogen Activator. A red line indicates an inhibitory pathway.
The components of the Fibrinolytic pathway comprise:
| Protein | Function | Half-Life | Gene |
|---|---|---|---|
| Plasminogen [PLG] - a 92-kDa protein - is synthesised as a zymogen and is the precursor of Plasmin, the active serine protease that is involved in the breakdown of Fibrin. Plasmin can also interact with other molecules e.g. Fibrinogen, Factor V, VIII. Native PLG is termed Glu-PLG as it contains a Glutamic Acid residue at the N-terminus |
PLG contains an active site serine that constitutes the active site of the serine protease Plasmin and in addition 5 kringle modules [so called because of their resemblance to a Scandinavian pastry, a Nordic variety of pretzel], 4 of which have Lysine binding sites and it through these that Plasminogen interacts with its substrates; its activators and its inhibitors. Cleavage of the Arg561-Val562 bond in PLG either by t-PA or u-PA converts the protein from an inactive zymogen to the active serine protease - Plasmin. |
Glu-Plasminogen has a T½ of ~50hr Lys-Plasminogen has a T½ of ~20hr. Plasma concentration 2µM [0.2mg/mL] |
PLG Maps to the long arm of Chromosome 6: 6q27 |
| t-PA | A 70-kDa serine protease secreted constitutively by vascular endothelial cells. T-PA is the principal activator of PLG. T-PA has a shortly half-life of 2-3 minutes in plasma due to the presence of a potent inhibitor termed PAI-1. The affinity of t-PA for PLG increases significantly in the presence of a fibrin clot and the formation of a ternary complex. Cleavage of t-PA from a single chain molecule to a two chain molecule is associated with an increase in its enzymatic activity although the single chain form has significant activity. |
T½ 2-3 minutes Plasma concentration 70pM [5ng/mL] |
PLAT Maps to the short arm of Chromosome 8. 8p11.1 |
| u-PA | U-PA, a 53-kDA protein - was first isolated from urine and from which it obtains its name. U-PA is an activator of PLG to Plasmin and primarily involved in extra-vascular remodelling and repair after tissue injury. It also has a role in mediating different types of immune response and in tumour metastasis. The single chain form of U-PA [scu-PA] is inactive but cleavage by Plasmin and other proteases generates a two chain form which is functionally active. |
T½ 2-3 minutes Plasma concentration 2-4 ng/mL |
PLAU Maps to the long arm of Chromosome 10: 10q22.2 |
| PAI-1 | A 50-kDA protein and the major inhibitor of t-PA and u-PA. Secreted constitutively by vascular endothelial cells. Also stored in the platelet. Member of the SERPIN [serine protease inhibitor] super-family of proteins. t-PA:PAI-1 complexes are removed by the liver. t-PA bound to a fibrin clot is relatively protected from inactivation by PAI-1 |
T½ 4-5 minutes | SERPINE1 Maps to the long arm of Chromosome 7: 7q22 |
| PAI-2 | Member of the SERPIN [serine protease inhibitor] super-family of proteins. Inhibitor of t-PA and u-PA. Present in many cells and is up-regulated during pregnancy as it is synthesised by the placenta. Significant levels of PAI-2 in plasma are found only in pregnancy. |
T½ 10-11 minutes | SERPINB2 Maps to long arm of Chromosome 18: 18q21.33-q22.1 |
| α2-antiplasmin [α2-Plasmin Inhibitor] |
A 63-kDa protein and the major inhibitor of Plasmin. Member of the SERPIN [serine protease inhibitor] super-family of proteins. Cross-linked into the fibrin clot by FXIIIa and so renders the clot resistant to fibrinolysis. |
T½ 72 hours Plasma concentration 1µM [70µg/mL] |
SERPINF2 Maps to the short arm of chromosome 17: 17p13 |
| Fibrinogen | Converted by Thrombin to Fibrin and cross-linked to form an insoluble polymer by FXIIIa | T½ 90 hours | |
| TAFI [Thrombin-Activatable Fibrinolysis Inhibitor] |
TAFI, a 60-kDA protein - is activated by Thrombin to TAFIa. TAFIa cleaves the C-terminal Lysine residues from Plasmin and therefore, the binding sites for both PLG and t-PA and as a result it down regulates Fibrinolysis. Thrombin is a relatively weak activator of TAFI, but in the presence of Thrombomodulin [Tm] the activation by Thrombin is increased by at least 1000-fold. |
T½ 10 minutes | CPB2 Maps to the long arm of Chromosome 13: 13q14.11 |
The individual assays for the various components of the Fibrinolytic pathway are covered in detail elsewhere on Practical-Haemostasis.com.
The diagram below outlines some of the possible causes of disordered Fibrinolysis.

Click HERE to return to the top of the page.
