Plasminogen Assays
Introduction
Plasminogen [Plg] is synthesised in the liver and circulates in two major forms:
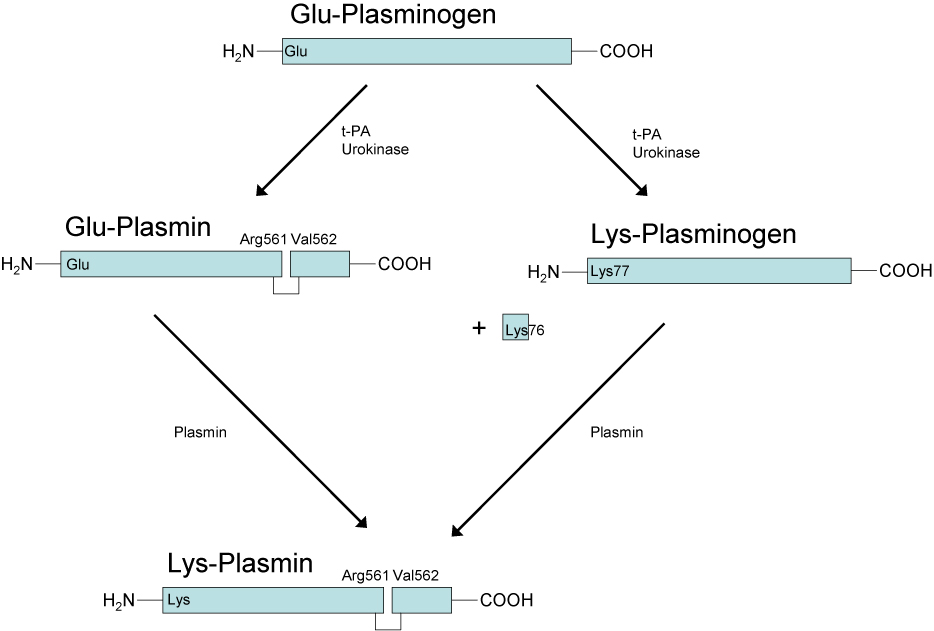
Glu-Plasminogen and Lys-Plasminogen. In its native form Plasminogen contains a Glutamic acid residue at the N-terminus and this molecule is termed Glu-Plasminogen. Activation of Glu-Plasminogen [via t-PA or u-PA] occurs through cleavage at Arg561 and Val562 to generate an active two chain [tc] molecule – Glu-Plasmin. The heavy chain contains the Lysine binding sites whilst the light chain has the serine protease activity. However, the Lysine binding sites in the heavy chain are buried and so this molecule is functionally inactive.
Auto-catalytic conversion of Glu-Plasmin to Lys-Plasmin occurs via cleavage of the heavy chain at Lys76-Lys-77 causing a significant conformational change in its structure and which exposes the Lysine binding sites and allows its binding to the fibrin clot.
Interestingly both Lys-Plasmin and Glu-Plasmin can cleave the Lys76-Lys-77 bond in Glu-Plasminogen to form Lys-Plasminogen.
Glu-Plasminogen has a T½ of ~50hr whereas Lys-Plasminogen has a T½ of ~20hr.
Plasmin - a serine protease is a key component of the Fibrinolytic pathway. It is converted to its active form, Plasmin, by tissue Plasminogen Activator (t-PA). The conversion of Plasminogen to Plasmin by t-PA is relatively inefficient in the absence of a fibrin clot but in the presence o Fibrin the efficiency of conversion increases significantly. Plasminogen can also be converted to Plasmin by u-PA [Urokinase-type Plasminogen Activator], Factor XIa and Factor XIIa.
Plasmin cleaves Fibrin into a number of soluble fibrin degradation products (FDPs). FDPs competitively inhibit Thrombin [FIIa] and so reduce conversion of Fibrinogen to Fibrin. Plasmin is responsible for both clot breakdown and, indirectly, for reducing clot formation.
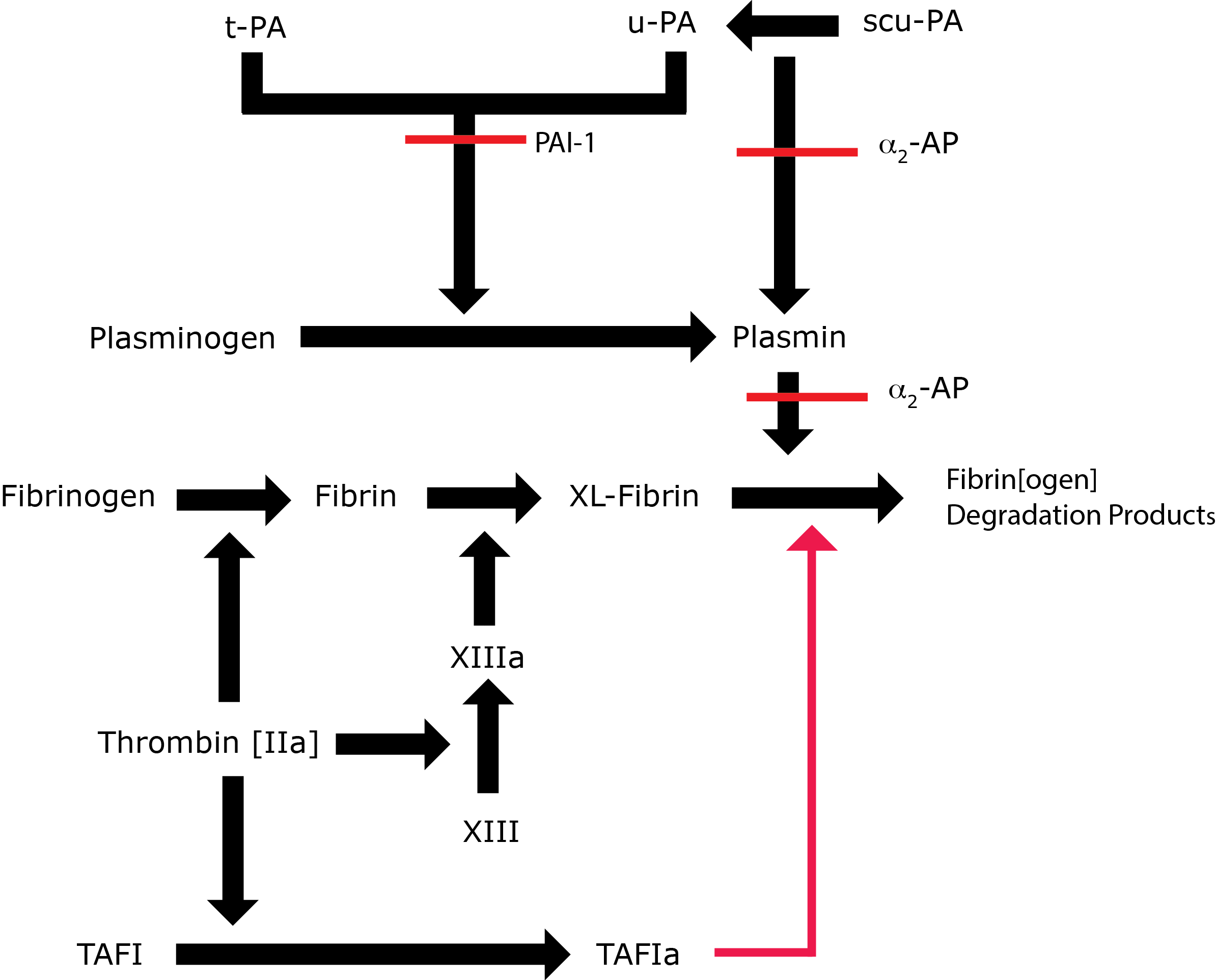
Key: t-PA - Tissue Plasminogen Activator; u-PA - Urokinase-type Plasminogen Activator; XL-Fibrin - cross-linked fibrin; TAFI - Thrombin Activatable Fibrinolytic Inhibitor; TAFIa - Activated Thrombin Activatable Fibrinolytic Inhibitor; α2-AP - Alpha-2-antiplasmin [Alpha2-Plasmin Inhibitor]; scu-PA - single chain Urokinase-type Plasminogen Activator. A red line indicates an inhibitory pathway.
In addition to its fibrinolytic role, Plasmin also cleaves Fibronectin, Thrombospondin, Laminin, von Willebrand factor, some collagenases and some components of the complement system. It has a role in ovulation and wound healing. For these reasons the activity of Plasmin is carefully regulated. Plasmin is inactivated by alpha 2-antiplasmin [α2-AP] also known as alpha 2-plasmin inhibitor [α2-PI] and may also be converted to Angiostatin, an angiogenesis inhibitor by an alternative pathway.
Plasminogen deficiency is inherited in an autosomal recessive manner. The paucity of cases makes any association with VTE unclear and published literature is conflicting on the thrombotic risk associated with Plasminogen deficiency.
There are two types of inherited Plasminogen deficiency:
| Type | Plasminogen Assays | Prevalence | Clinical Association |
|---|---|---|---|
| I | Decreased | ~ 1:1.6 million | Associated with pseudomembrane disease especially ligneous conjunctivitis, impaired wound healing and possibly reduced female fertility. |
| II | Decreased functional activity but normal immunological activity | Up to 3% in some ethnic groups | No pseudomembranes but impaired wound healing and probably reduced female fertility |
Principles & Methods
The assays for Plasminogen reflect the different potential defects.
1. Immunological Assays:A variety of immunological methods exist for measuring Plasminogen antigenic levels including ELISA assays.
2. Functional Assays: Plasminogen activity can be assayed by its activation to Plasmin [by either Streptokinase or U-PA] and then the measuring the functional activity of Plasmin using a variety of substrates.
Chromogenic Plasminogen assays: These are commonly used as a semi-functional assessment of Plasminogen activity.
Plasminogen is activated in most assays by Streptokinase which binds to Plasminogen converting it to Plasmin. The Plasmin cleaves a specific chromogenic substrate and the colour change is directly proportional to Plasminogen.
In patients with elevated FDPs an over estimation of Plasminogen may occur as the FDPs stimulates the assay. This can be overcome by the addition of Plasminogen-free human Fibrinogen to the samples.
Interpretation
A functional Plasminogen assay should be performed first. If this is normal then the patient is very unlikely to have plasminogen deficiency. If the functional test indicates reduced activity then antigen testing can be undertaken to distinguish Type I and II disorders.
The table below summarises the causes of test abnormalities.
| Increased Plasminogen Levels | Decreased Plasminogen Levels |
|---|---|
| Anabolic steroids Hypothyroidism Hormonal contraceptives Obesity Pregnancy Plasminogen is an acute phase protein and so the levels are increased in association with infection, trauma, surgery, inflammation and malignancy. |
1. Hereditary deficiencies 2. Acquired deficiencies Tranexamic acid L-Asparaginase Disseminated Intravascular Coagulation [DIC] Liver disease During and after thrombolytic therapy The newborn infant Elevated HRG levels Sepsis Hyperthyroidism |
Abnormal tests should always be verified by repeat and deficiency should not be diagnosed on the basis of a single abnormal result.
Reference Ranges
Plasminogen reference activity: 0.75 - 1.60 U/mL
Plasminogen antigen: 150 - 250 ng/L.
The reference ranges may vary with ethnicity and are reported to be higher in African males.
Plasminogen levels are reduced in the newborn.
What Test Next
Family testing may be appropriate to permit appropriate counselling.
In cases of apparent hereditary Plasminogen deficiency, mutational analysis of the PLG genes is essential.
